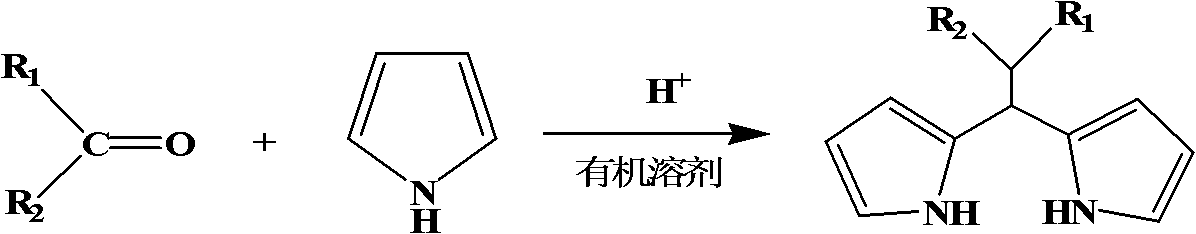
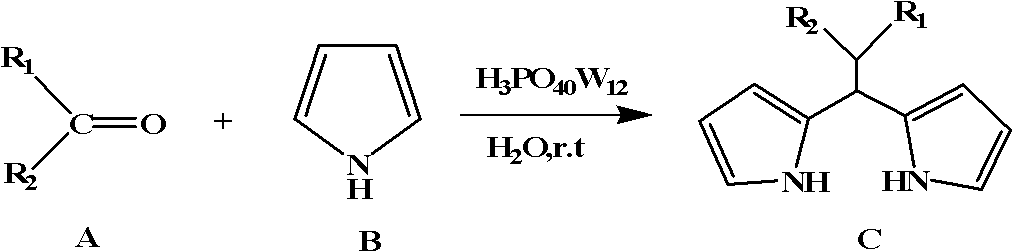Dipyrromethene compound synthetic method
A technology of dipyrrolemethane and a synthesis method, which is applied in the field of synthesis of dipyrrolemethane compounds, can solve the problems of difficult operation, harsh conditions, complicated post-processing process and the like, and achieves a high degree of greening, simple post-processing and low cost. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
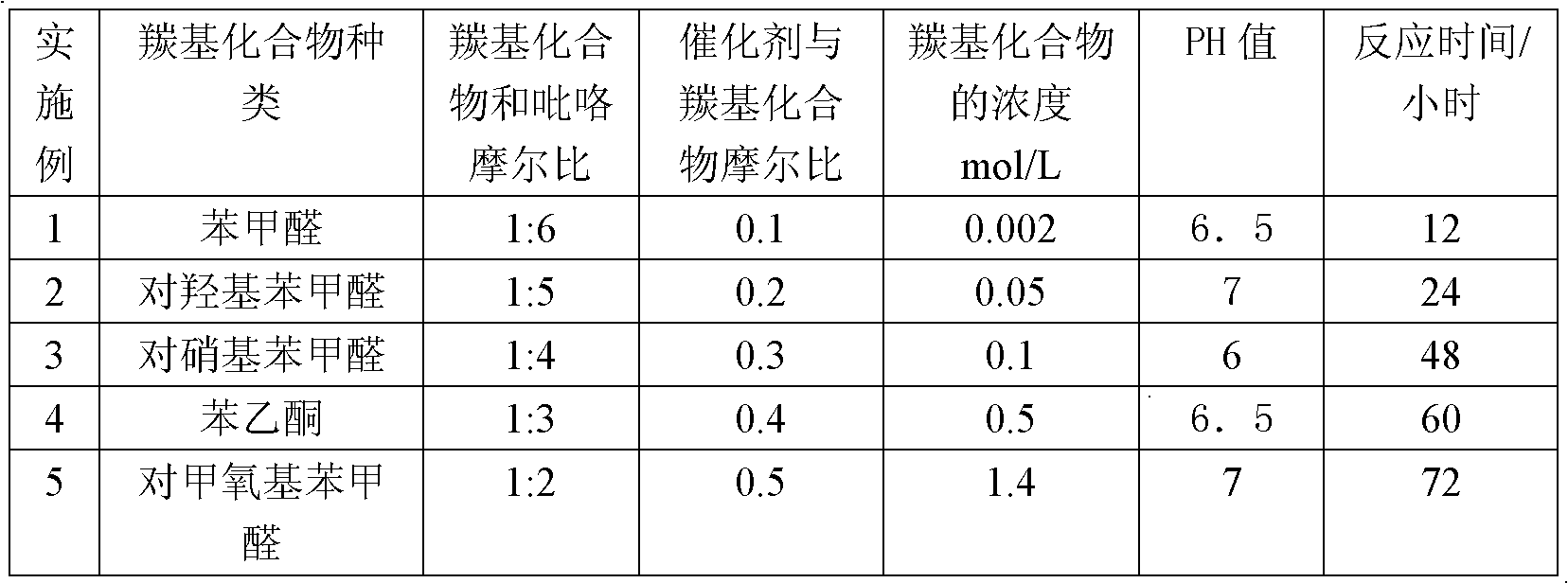
[0013] Example 1 Add 50 mL of deionized water, 0.5 mL (5 mmol) of benzaldehyde and 2 mL (30 mmol) of pyrrole into a three-necked flask with a dropping device, and the concentration of benzaldehyde in the reaction system is 2 × 10 -3 ~1.4mol / L; purge the solution with nitrogen for 10 minutes to remove oxygen in the water; add 1.4g (0.15mmol) phosphotungstic acid and seal the system, under nitrogen protection, the reaction temperature is 25°C, stir, react for 12 hours, add an appropriate amount of ammonia water The pH of the solution was set at 6.5 to quench the reaction, suction filtered, the filter cake was washed 5 times with distilled water, and vacuum-dried at 40° C. to obtain 0.91 g of 5-phenyldipyrromethane with a yield of 82%.
[0014] The structural characterization data of the product: mp=100°C, 1 H NMR (400MHz, CDCl 3 ): δ5.45(br s, 1H, mesoH), 5.90(m, 2H, 2C 3 -H), 6.14 (dd, 2H, J=2.8, 5.8, 2C 4 -H), 6.67 (br d, 2H, J=2.8, 2C 5 -H), 7.20 (m, 2H, H-Ar), 7.25 (m, 2...
Embodiment 2
[0017] The process conditions of Example 2 are shown in Table 1, and the rest are the same as in Example 1 to obtain 0.65 g of 5-(4-hydroxyphenyl) dipyrromethane with a yield of 55%.
[0018] Structural characterization data of the product: mp = 158°C. 1 HNMR (400MHz, CDCl 3 ): δ5.40(s, 1H, mesoH), 5.90(m, 2H, 2C 3 -H), 6.14 (dd, 2H, J=2.8, 5.8, 2C 4 -H), 6.67(m, 2H, 2C 5 -H), 6.75 (d, 2H, J=8.5, H-Ar), 7.05 (d, 2H, J=8.5, Ar-H), 7.96 (br s, 2H, N-H).
Embodiment 3
[0019] The process conditions of Example 3 are shown in Table 1, and the rest are the same as in Example 1 to obtain 1.3 g of 5-(4-nitrophenyl)dipyrromethane with a yield of 95%.
[0020] Characterization data: mp = 159°C. 1 H NMR (400MHz, CDCl 3 ): δ5.58(s, 1H, mesoH), 5.87(d, 2H, J=5.7Hz, 2C 3 -H), 6.17 (dd, 2H, J=2.8, 5.7, 2C 4 -H), 6.74 (dd, 2H, J=2.8, 1.2, 2C 5 -H), 7.36(d, 2H, J=8.6, H-Ar), 7.98(br s, 2H, N-H), 8.16(d, 2H, J=8.6, Ar-H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com