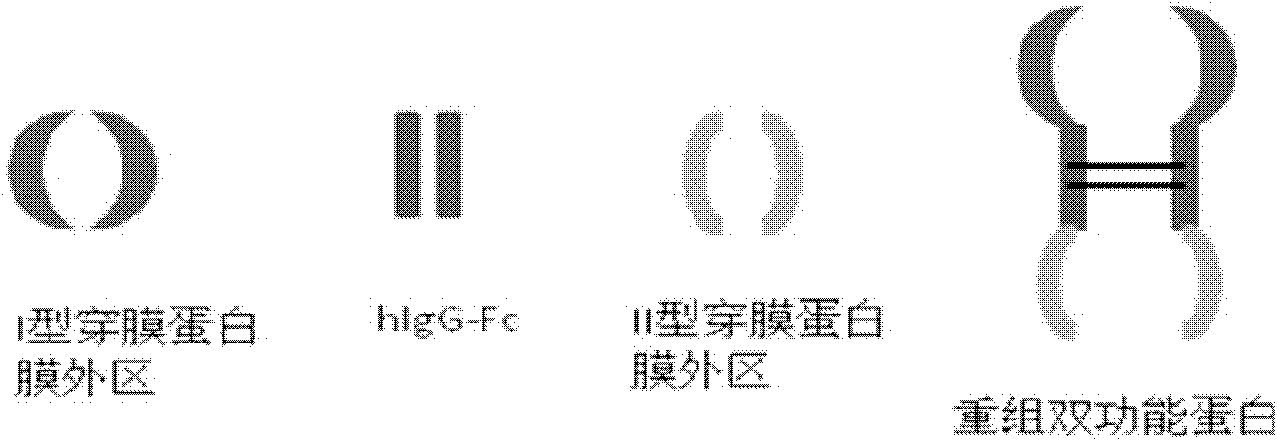
Novel recombined dual-function fusion protein and its preparation method and application
A protein and membrane-penetrating protein technology, applied in the field of biomedicine, can solve the problem of no recombinant bifunctional fusion protein drug, and achieve the effect of inhibiting migration and invasion
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
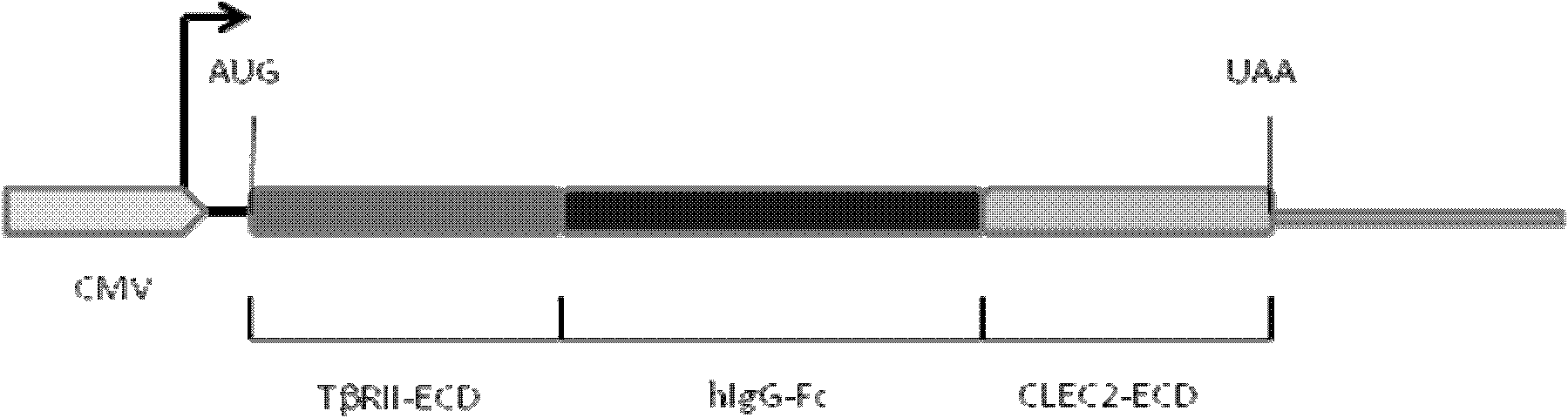
[0122] Construction of pIg-TβRII-Fc-CLEC2 vector
[0123] 1. Improve the pIg-Fc vector and remove the stop codon at the Fc carboxy-terminus.
[0124] Using primers 1 (SEQ ID NO: 1) and 2 (SEQ ID NO: 2) (see Table 1) to amplify the Fc-encoding gene, the amplified product was cloned into EcoRI and XhoI of the original pIg-Fc vector (purchased from CLONETECH) Cloning site.
[0125] 2. PCR amplification of the extramembrane region of type II TGF-β receptor.
[0126] Using primers 3 (SEQ ID NO: 3) and 4 (SEQ ID NO: 4), using cDNA derived from breast cancer cell line (MDA-MB-231) (Cell Bank of Chinese Academy of Sciences) as a template, PCR amplified type II TGF- The extramembrane region of the β receptor, the amplified product was cloned into the HindIII and EcoRI cloning sites of the improved pIg-Fc vector to form pIg-TβRII-Fc.
[0127] 3. PCR amplification of the extramembrane region of CLEC-2
[0128] Using primers 5 (SEQ ID NO: 5) and 6 (SEQ ID NO: 6), using cDNA derived fr...
Embodiment 2
[0136] Expression of TβRII-Fc-CLEC2
[0137] Use the method of small-scale transient transfection to detect whether the pIg-TβRII-Fc-CLEC2 expression vector expresses recombinant protein: 5x10 4 CHO cells were plated in each well of a 24-well plate containing 0.5 ml of DMEM medium and incubated at 37°C in 5% CO 2 cultured in culture phase for 24 hours. During transfection, a certain amount of carrier DNA and transfection reagent (Lipofactamine2000, Invitrogen) were uniformly mixed together (the specific method can be carried out in accordance with the steps provided by the manufacturer) and added to each well after standing at room temperature for 20 min, and continued to cultivate 24 hours. At this time, a certain amount of culture supernatant was taken out to detect protein expression by enzyme-linked immunosorbent assay.
[0138] If the anti-Fc antibody is used for hybridization, the bifunctional fusion protein TβRII-Fc-CLEC2 and the monofunctional fusion protein TβRII-F...
Embodiment 3
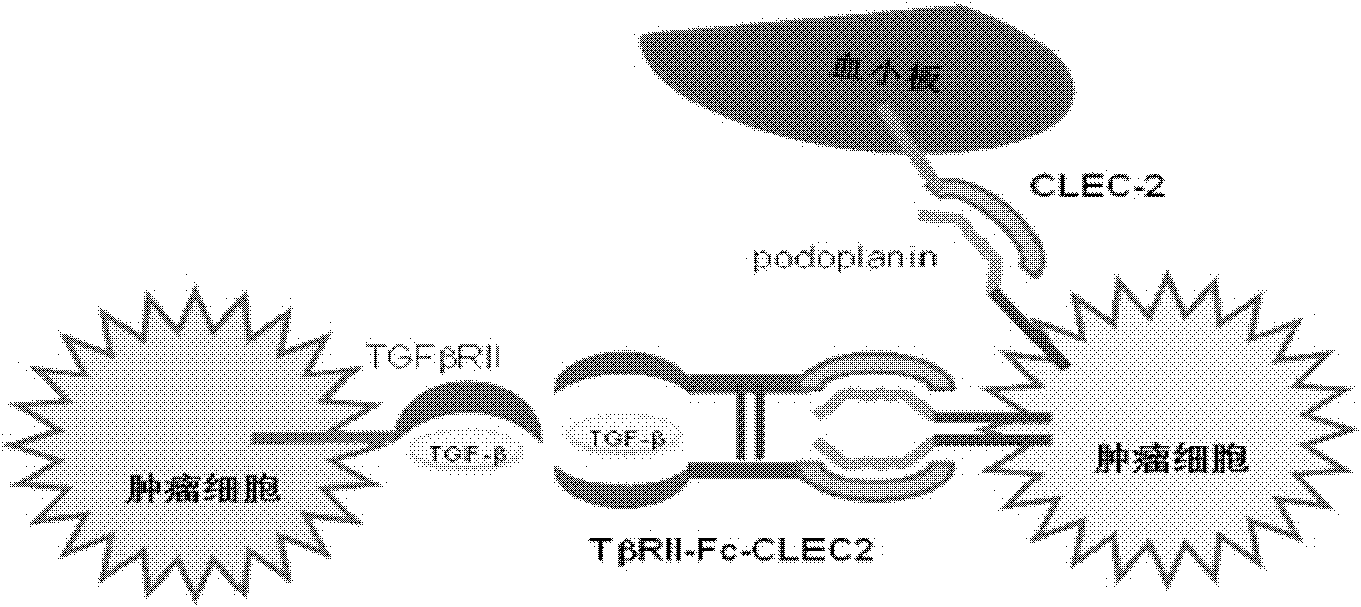
[0141] TβRII-Fc-CLEC2 can simultaneously bind to two targets (ligands)
[0142] Using enzyme-linked immunosorbent assay (ELISA) method and flow cytometry detection method, the binding properties of the fusion protein and the two targets (ligands) were determined.
[0143] Detection method: Coat the ELISA plate with TGF-β1 at 4°C overnight, add the doubling dilution (from 200nM to 0.1nM of TβRII-Fc-CLEC2 to the ELISA plate coated with TGF-β1, incubate at room temperature for one hour, After washing, add horseradish peroxidase-labeled anti-Fc fragment antibody and continue to incubate for one hour, then wash again. Add an appropriate amount of horseradish peroxidase substrate for color development and use a microplate reader to detect the results.
[0144] Figure 5 A shows that the ELISA test has confirmed that TβRII-Fc-CLEC2 can be combined with human TGF-β1, and 50% effective binding concentration (ED 50 ) is about 3nM.
[0145] Figure 5 B shows that the results of flow ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com