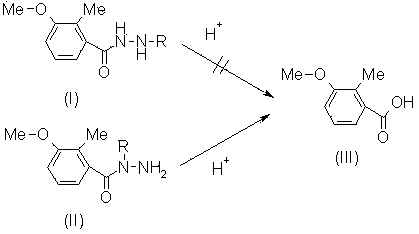
Purifying method of N-(3-methoxy-2-methyl benzoyl)-N'-tert-butylhydrazine
A technology of methylbenzoyl and methylbenzoyl chloride, which is applied in the field of compound preparation, can solve problems such as poor separation effect, high cost, and non-environmental protection, and achieve the effects of reducing three wastes, improving purity, and improving product purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] a. Preparation of crude product:
[0023] In a 300L reactor equipped with a stirring paddle, add 30kg of tert-butylhydrazine hydrochloride and 100L of toluene, stir, cool down, and add 20% liquid caustic soda dropwise at 0°C. After dropping, 10kg of 3-methoxy-2-methylbenzoyl chloride and 20% liquid caustic soda are added dropwise at this temperature. After dropping, stir at room temperature for five hours, then let stand to separate the water layer and remove the organic layer, a white solid was obtained.
[0024] B, the purification of N-(3-methoxy-2-methylbenzoyl)-N'-tert-butylhydrazine:
[0025] In a 300L reactor equipped with a stirring paddle, add 11.8kg of the crude product obtained in step a (containing 85% of compound (I), 10% of compound (II), etc.), add 200L of water, and add concentrated hydrochloric acid dropwise at room temperature When the pH is less than 1, raise the temperature to 100°C, keep it warm for 1 hour, cool down to room temperature, and filte...
Embodiment 2
[0027] In a 300 L reactor equipped with a stirring paddle, add 11.8 kg of the crude product obtained in step a of Example 1, (containing 85% of compound (I), 10% of compound (II), etc.), add 200 L of water, and Concentrated hydrochloric acid was added dropwise until the pH was equal to 2, and the temperature was raised to 100°C, kept for 1 hour, cooled to room temperature, and filtered to obtain 0.7kg of 3-methoxy-2-methylbenzoic acid (purity 98% by HPLC analysis). In the mother liquor, liquid caustic soda is added dropwise to PH10, filters, obtains 10.2kg high-purity N-(3-methoxyl group-2-methylbenzoyl)-N'-tert-butylhydrazine (purity through HPLC analysis is 96 %), the yield is 97.6%.
Embodiment 3
[0029] In a 300 L reactor equipped with a stirring paddle, add 11.8 kg of the crude product obtained in step a of Example 1, (containing 85% of compound (I), 10% of compound (II), etc.), add 200 L of water, and Add concentrated hydrochloric acid dropwise until the pH is less than 1, raise the temperature to 100°C, keep it warm for 0.5 hours, cool down to room temperature, and filter to obtain 0.8kg of 3-methoxy-2-methylbenzoic acid (purity 98% by HPLC analysis). In the mother liquor, liquid caustic soda is added dropwise to PH9, filters, obtains 9.9kg high-purity N-(3-methoxy-2-methylbenzoyl)-N'-tert-butylhydrazine (the HPLC analysis purity is 98 %), the yield is 96.7%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com