Nitrogen substitution podophyllum type derivatives with antitumor activity and preparation method and application thereof
A technology of anti-tumor activity and nitrogen substitution, applied in the preparation and application of podophyllotoxin derivatives, nitrogen-substituted podophyllotoxin derivatives in the preparation of anti-tumor drugs, nitrogen-substituted podophyllotoxin derivatives and their preparation fields, can Solve the problems of limited use and low anti-tumor activity, and achieve the effect of improved anti-tumor activity and good anti-tumor efficacy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
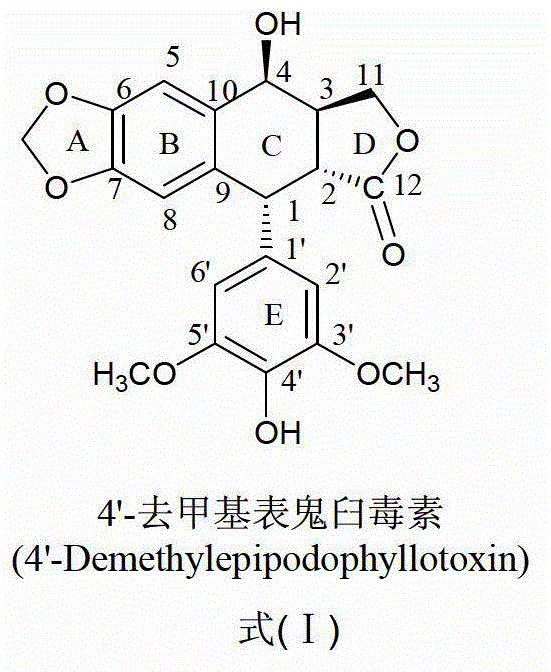
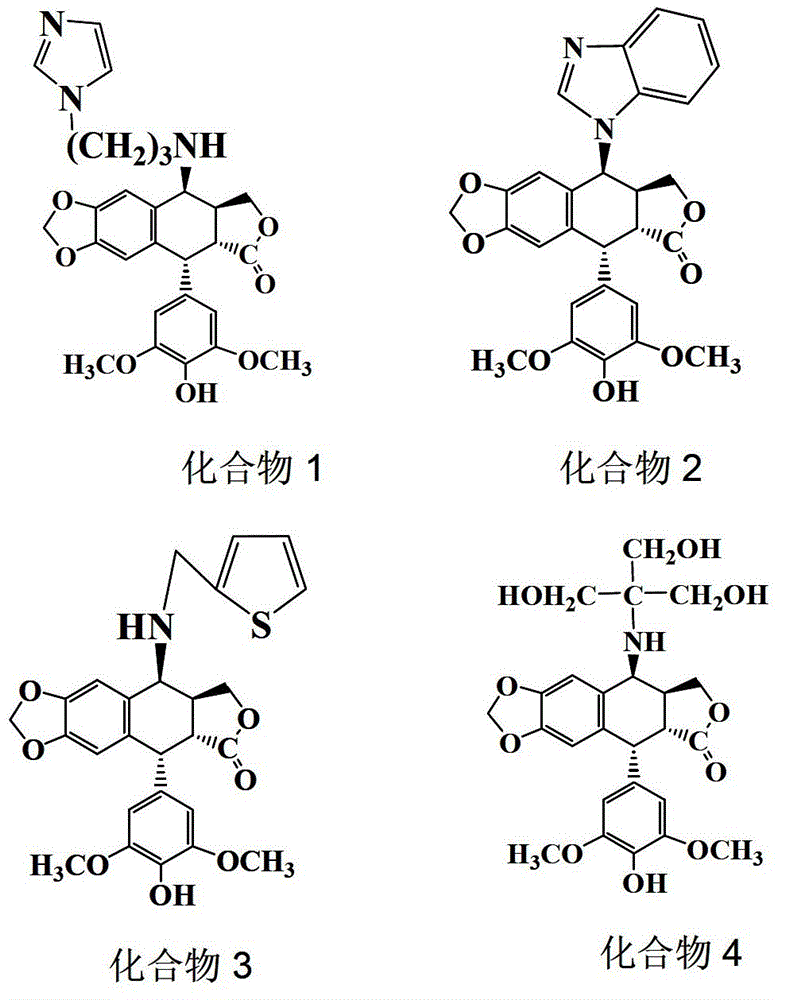
[0040] Example 1 Synthesis and purification of 4-N-(1-(3-aminopropyl)imidazole-1)-4-deoxy-4'-desmethyl epipodophyllotoxin (compound (1))
[0041] (1) Synthesis of 4-N-(1-(3-aminopropyl)imidazole-1)-4-deoxy-4'-desmethyl epipodophyllotoxin: Weigh 1mol 4'-desmethyl epipodophyllotoxin The activated product at the 4-position of the C ring of the toxin (prepared in Preliminary Example 1) was vacuum-dried at 45°C for 2 hours; ) into a four-neck flask, add the dried activated product of 4′-demethylepipodophyllotoxin C ring 4 and 3mol 1-(3-aminopropyl)imidazole, stir and react at 80°C for 48 hours, and the reaction After the liquid was spin-dried, the crude product of 4-N-(1-(3-aminopropyl)imidazole-1)-4-deoxy-4'-desmethyl epipodophyllotoxin was obtained.
[0042] (2) Isolation and purification of 4-N-(1-(3-aminopropyl)imidazole-1)-4-deoxy-4'-desmethyl epipodophyllotoxin:
[0043] Separation and purification using silica gel column chromatography and gel column chromatography:
[00...
Embodiment 2
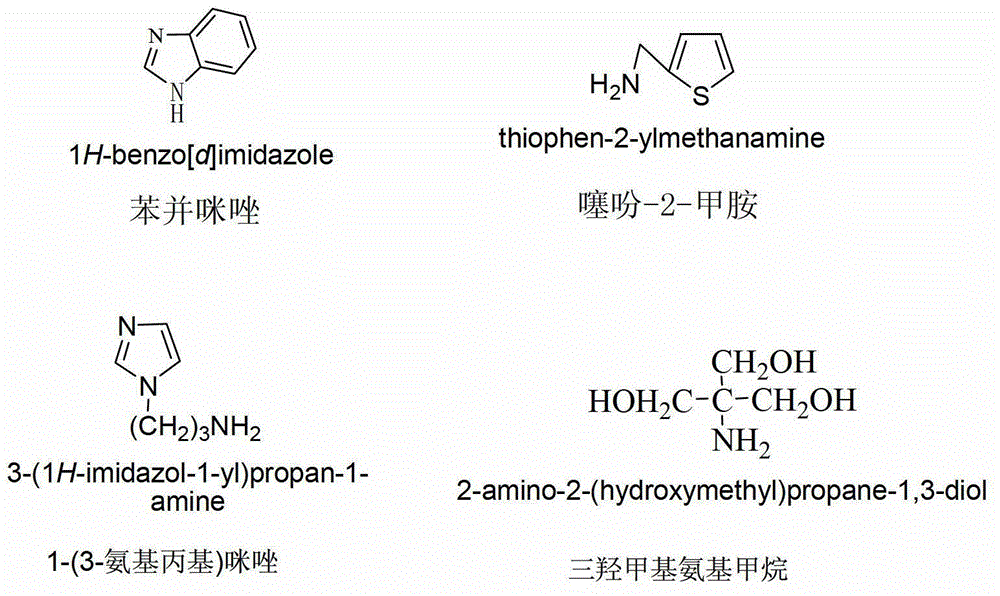
[0047] Example 2 Synthesis and purification of 4-N-(benzimidazole)-4-deoxy-4'-desmethyl epipodophyllotoxin (compound (2))
[0048] (1) Synthesis of 4-N-(benzimidazole)-4-deoxy-4'-desmethyl epipodophyllotoxin: Weigh 1mol of 4'-desmethyl epipodophyllotoxin C ring 4 activation product ( Prepared in Preparatory Example 1), vacuum-dry at 45°C for 2 hours; under nitrogen protection, add dried acetonitrile (the drying method is the same as that of dichloromethane in Preparatory Example 1) into a four-necked bottle, and add the dried The activated product of the 4th position of the C ring of 4'-desmethyl epipodophyllotoxin and 3mol benzimidazole were stirred and reacted at 80°C for 48 hours; after the reaction was completed, the reaction solution was spin-dried, and dissolved by adding 50ml of ethyl acetate and then cleaned with an ultrasonic cleaner Ultrasound for 5 minutes, there will be insoluble matter at this time, and the insoluble matter obtained after filtering with filter pap...
Embodiment 3
[0054] Example 3 Synthesis and purification of 4-N-(2-thienylamine)-4-deoxy-4'-desmethyl epipodophyllotoxin (compound (3))
[0055] (1) Synthesis of 4-N-(2-thiophenemethylamine)-4-deoxy-4′-desmethyl epipodophyllotoxin: Weigh 1 mol of 4′-desmethyl epipodophyllotoxin C ring 4 activation The product (prepared in Preliminary Example 1) was vacuum-dried at 45°C for 2 hours; under nitrogen protection, the dried acetonitrile (drying method was the same as that of dichloromethane in Preparatory Example 1) was added to a four-necked flask, and dried A good 4′-demethyl epipodophyllotoxin C-ring 4-activated product and 3mol thiophene-2-methylamine (80°C stirred and reacted for 48 hours, and the reaction solution was spin-dried to obtain the crude product
[0056] (2) Separation and purification:
[0057] Separation and purification using silica gel column chromatography and gel column chromatography:
[0058] (A) Use a normal phase silica gel column (normal phase silica gel: China Qing...
PUM
| Property | Measurement | Unit |
|---|---|---|
| The inside diameter of | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com