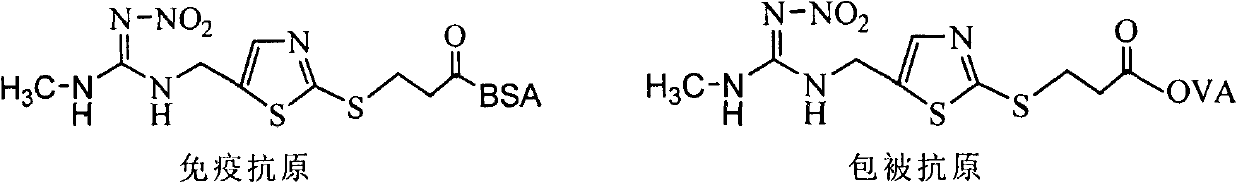Clothianidin antigen, antibody and application thereof
A clothianidin and antibody technology is applied in the field of immunochemical analysis to achieve the effects of large analysis capacity, high accuracy, and easy popularization and promotion.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
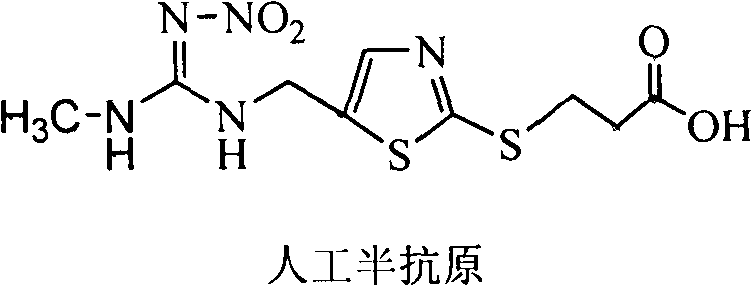
[0025] 1 Synthesis of artificial hapten
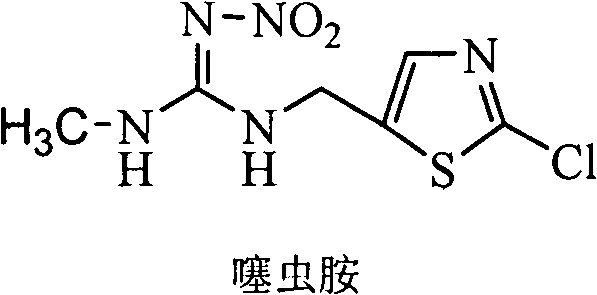
[0026] Substituting the chlorine atom on the thiazole ring of the clothianidin molecule to synthesize a hapten with a four-atom connecting arm, which not only retains the characteristic group of clothianidin to the greatest extent, but also forms a carboxyl terminal group, which can better follow the protein coupling. The product was purified by mass spectrometry (ESI) and H NMR spectroscopy ( 1 H-NMR) identification. The chemical name of clothianidin artificial hapten is 3-(5-((3-methyl-2-nitroguanidine)-ylmethyl)thiazole-2-mercapto)propionic acid, and its molecular structure is as follows:
[0027]
[0028] 1.1 Synthesis of artificial haptens
[0029] Dissolve 8mmol (0.45g) of KOH in 20mL of ethanol, add 4mmol (0.42g) of β-mercaptopropionic acid and stir, after complete dissolution, add 4mmol (1.02g) clothianidin, stir and reflux at 80°C for 2h. After the reaction, the reaction solution was filtered, and the filtrate was conce...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com