Difluoroalkyl itaconate and its preparation method
A technology for bisfluoroalkyl itaconate and itaconic acid is applied in the field of bisfluoroalkyl itaconate and its preparation, which can solve problems such as prohibition and cumulative toxicity, and achieve mild preparation process conditions and low equipment requirements. , the effect of high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Preparation of bis(1,1,5-trihydrooctafluoropentyl) itaconate
[0038] In a reactor equipped with stirring, reflux condenser, water separator, and thermometer, add 150 g of cyclohexane, 26.0 g of itaconic acid, 92.8 g of 1,1,5-trihydrooctafluoropentanol, and hydroquinone 1.9 g and p-toluenesulfonic acid 3.0 g. Stir and heat to 81°C under the protection of nitrogen, react for 6 hours until no more water is formed, and about 6.5 g of water is separated to stop the reaction.
[0039] After the reaction mixture is cooled, filter to obtain a clear filtrate, wash with water until neutral, add 10 g of desiccant to dry for 6 hours, filter to remove the desiccant after drying, and distill off the solvent under reduced pressure from the filtrate at 50°C and a vacuum of 1333.0Pa to obtain a light yellow transparent The oily product is bis(1,1,5-trihydrooctafluoropentyl) itaconate, and the reaction yield is 85.6%. The product structural formula is as follows:
[0040]
[0041]...
Embodiment 2
[0045] Preparation of bis(1,1,2,2-tetrahydrotridecafluorooctyl) itaconate
[0046] In a reactor equipped with stirring, reflux condenser, water separator, and thermometer, add 200 g of toluene, 26.0 g of itaconic acid, 145.6 g of 1,1,2,2-tetrahydrotridecafluorooctyl alcohol, and 1.9 g of phenol and 3.0 g of p-toluenesulfonic acid. Stir and heat to 112°C, react for 8 hours until no more water is formed, and about 7.2 g of water is separated to stop the reaction.
[0047] After the reaction mixture is cooled, filter to obtain a clear filtrate, wash with water until neutral, add 15g of desiccant anhydrous sodium sulfate to dry for 8 hours, filter to remove the desiccant after drying, and distill the filtrate to remove the solvent under reduced pressure at 60°C and vacuum degree of 666.5Pa Bis(1,1,2,2-tetrahydrotridecafluorooctyl) itaconate was obtained as a light yellow transparent oil product, and the reaction yield was 73.2%. The product structural formula is as follows:
[...
Embodiment 3
[0053] Preparation of bis(1,1,3-trihydrotetrafluoropropyl) itaconate
[0054] In a reactor equipped with stirring, reflux condenser, water separator, and thermometer, add 100 g of benzene, 26.0 g of itaconic acid, 52.8 g of 1,1,3-trihydrotetrafluoropropanol, and 2.5 g of hydroquinone and 3.0 g of p-toluenesulfonic acid. Stir and heat to 80°C under the protection of nitrogen, react for 5 hours until no more water is formed, separate about 6.0 g of water, and stop the reaction.
[0055] After the reaction mixture is cooled, filter to obtain a clear filtrate, wash with water until neutral, add 5g of desiccant to dry for 5 hours, filter to remove the desiccant after drying, and distill the filtrate under reduced pressure at 50°C and a vacuum of 1333.0Pa to obtain a light yellow transparent The oily product is bis(1,1,3-trihydrotetrafluoropropyl) itaconate, and the reaction yield is 88.6%. The product structural formula is as follows:
[0056]
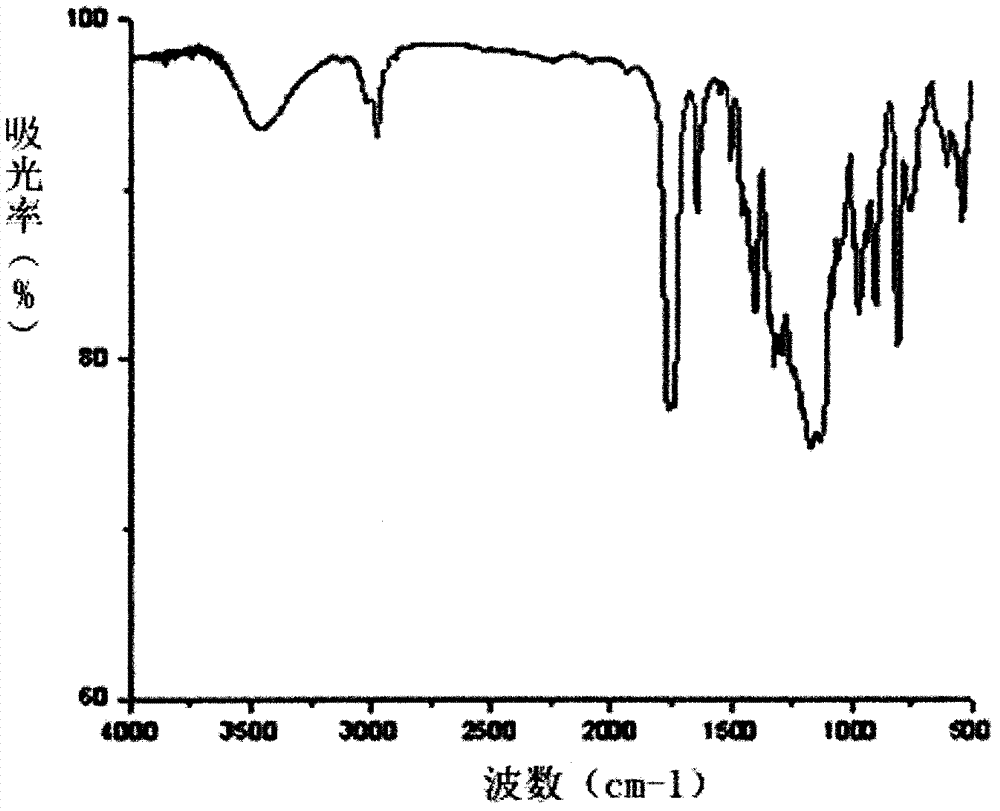
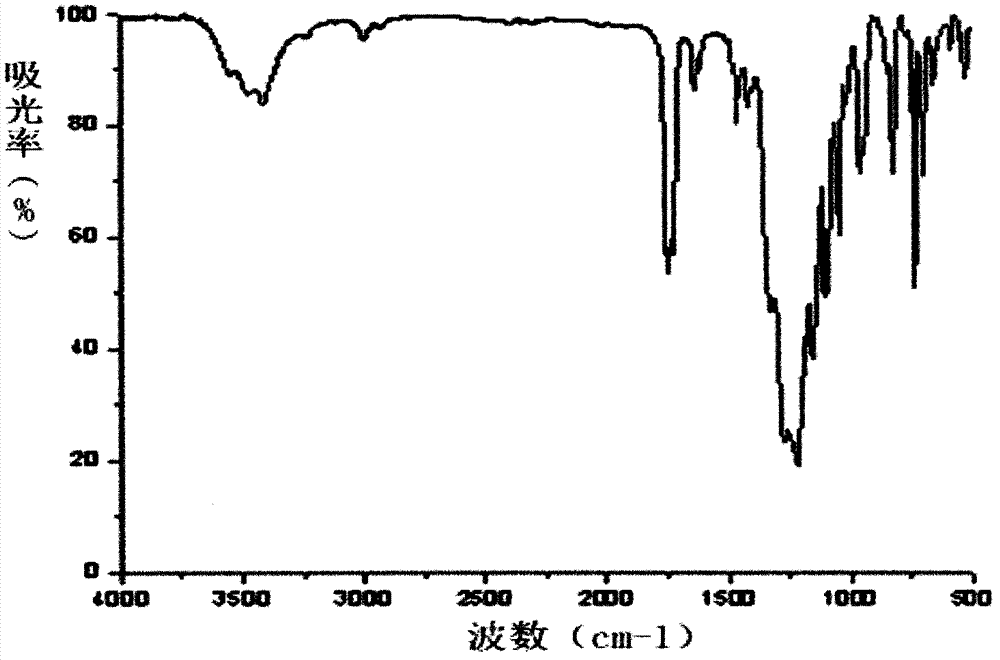
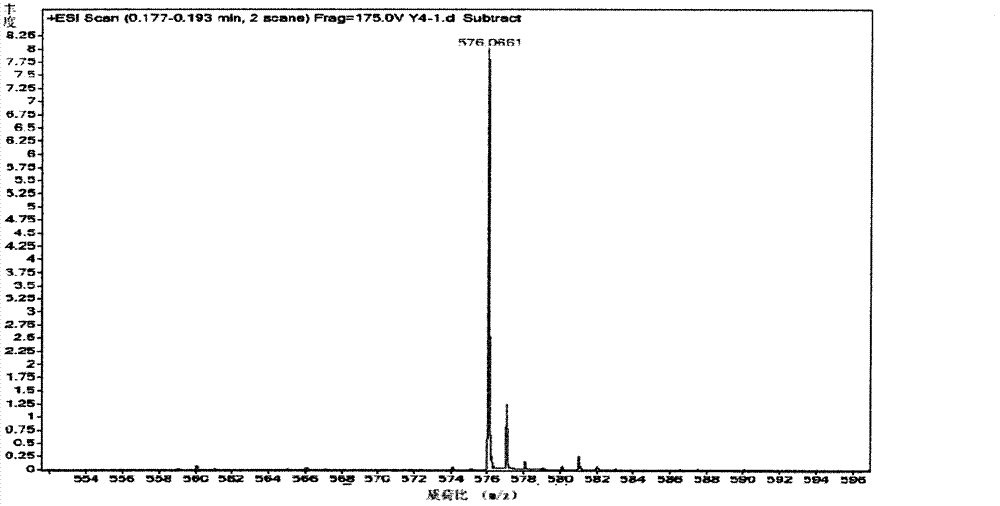
[0057] Product structure chara...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com