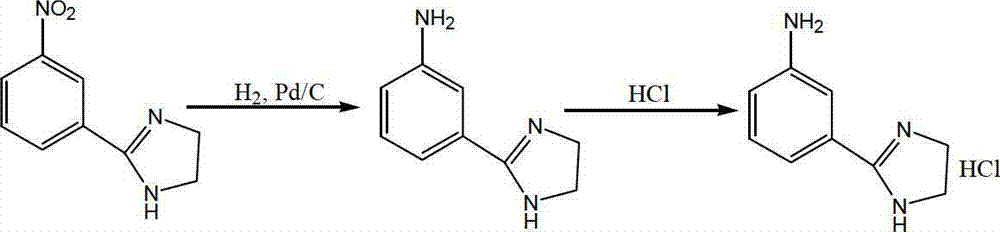
Preparation method of 2-(3-aminophenyl) imidazoline hydrochloride
A technology of imidazoline hydrochloride and aminophenyl, which is applied in the field of preparation of 2-imidazoline hydrochloride, can solve the problems of limited industrial production scale, difficult separation of material and liquid, complicated preparation process and the like, and achieves good industrialization prospects , Overcome the cumbersome operation and the effect of environmental friendliness
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] A kind of preparation method of 2-(3-aminophenyl) imidazoline hydrochloride, the steps are as follows:
[0032] Dissolve 124.4 kg of 2-(3-nitrophenyl) imidazoline in 600 L of water and add it to the hydrogenation kettle, then add 10.0 kg of palladium-carbon catalyst containing 5 wt% palladium, seal the hydrogenation kettle, and pass nitrogen gas 3 times to drain Air and check air tightness, replace nitrogen with hydrogen for 3 times, keep the pressure constant to 0.3MPa, control the reaction temperature at 50°C to 55°C, carry out reduction hydrogenation reaction for 3 hours, replace hydrogen with nitrogen for 3 times, filter the reaction solution, and recover palladium Carbon, take the light yellow filtrate, adjust the pH to 2.0 with 6mol / L hydrochloric acid, cool down to 0°C, let stand for crystallization for 1h, filter to obtain a white solid, and vacuum-dry at 60°C for 8 hours to obtain 122.1kg of an off-white solid.
[0033] After testing, the mp (melting point) is ...
Embodiment 2
[0035] A kind of preparation method of 2-(3-aminophenyl) imidazoline hydrochloride, the steps are as follows:
[0036] 126.5 kg of 2-(3-nitrophenyl) imidazoline was dissolved in 600L ethanol and added to the hydrogenation kettle, and then 10.0 kg of palladium-carbon catalyst containing 5 wt % of palladium was added, and the palladium-carbon catalyst containing 5 wt % of palladium was In order to adopt the method of Example 1 to recycle the catalyst after 5 times, seal the hydrogenation tank, pass nitrogen 3 times to discharge the air and check the air tightness, replace the nitrogen with hydrogen 3 times, keep the constant pressure to 0.01MPa, and control the reaction The temperature is 30°C to 35°C, carry out the reduction hydrogenation reaction for 6 hours, replace the hydrogen with nitrogen for 3 times, filter the reaction solution, recover palladium carbon, take the light yellow filtrate, adjust the pH to 2.0 with 6mol / L hydrochloric acid, and cool down to -2°C , standing ...
Embodiment 3
[0039] A kind of preparation method of 2-(3-aminophenyl) imidazoline hydrochloride, the steps are as follows:
[0040] 2-(3-nitrophenyl) imidazoline 125.0kg is dissolved in 600L water and adds in the hydrogenation kettle, then adds palladium carbon catalyst 10.0kg containing palladium amount 5wt%, the palladium carbon catalyst containing palladium amount 5wt% is Adopt the method of Example 1 to recycle the catalyst after 7 times, seal the hydrogenation tank, pass nitrogen 3 times to discharge the air and check the air tightness, replace the nitrogen with hydrogen 3 times, keep the constant pressure to 0.1MPa, and control the reaction temperature at 40°C to 45°C, carry out reductive hydrogenation reaction for 4.5 hours, replace hydrogen with nitrogen for 3 times, filter the reaction solution, recover palladium carbon, take light yellow filtrate, adjust pH=2.0 with 6mol / L hydrochloric acid, cool to 3°C, and statically Set aside for crystallization for 1 h, filter to obtain a whi...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com