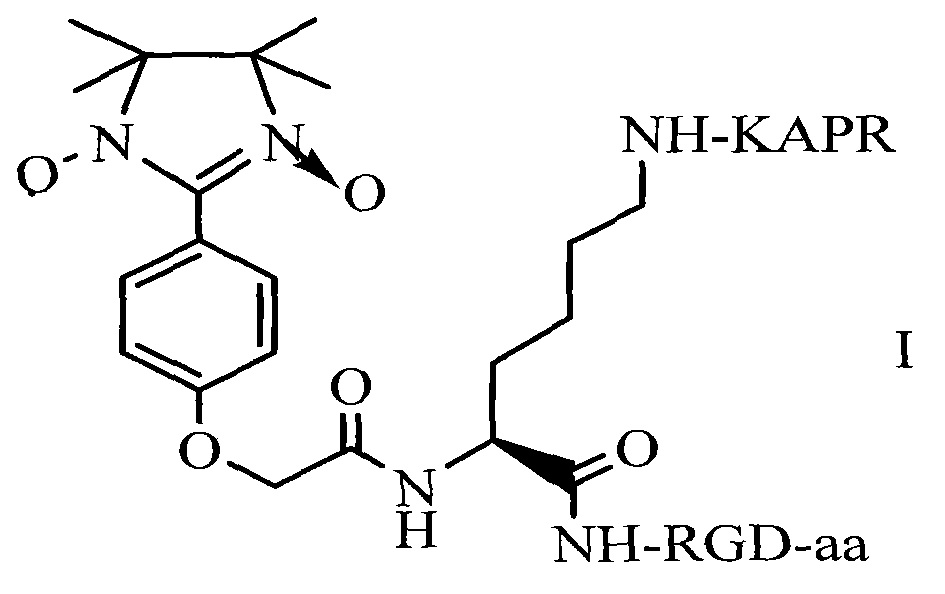
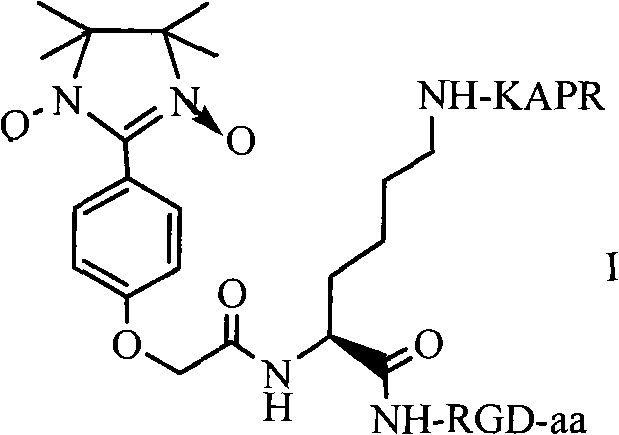
RPAK/imidazolidine/RGD ternary conjugate, preparation method and uses thereof
A technology of tetramethylimidazoline and compounds, applied in the field of RPAK/imidazoline/RGD ternary conjugates and their preparation and application, can solve the problem of inability to effectively cross the blood-brain barrier, complex, and limited curative effect in patients with cerebral infarction to achieve excellent anti-stroke activity, protect neurological function, and reduce the volume of cerebral infarction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
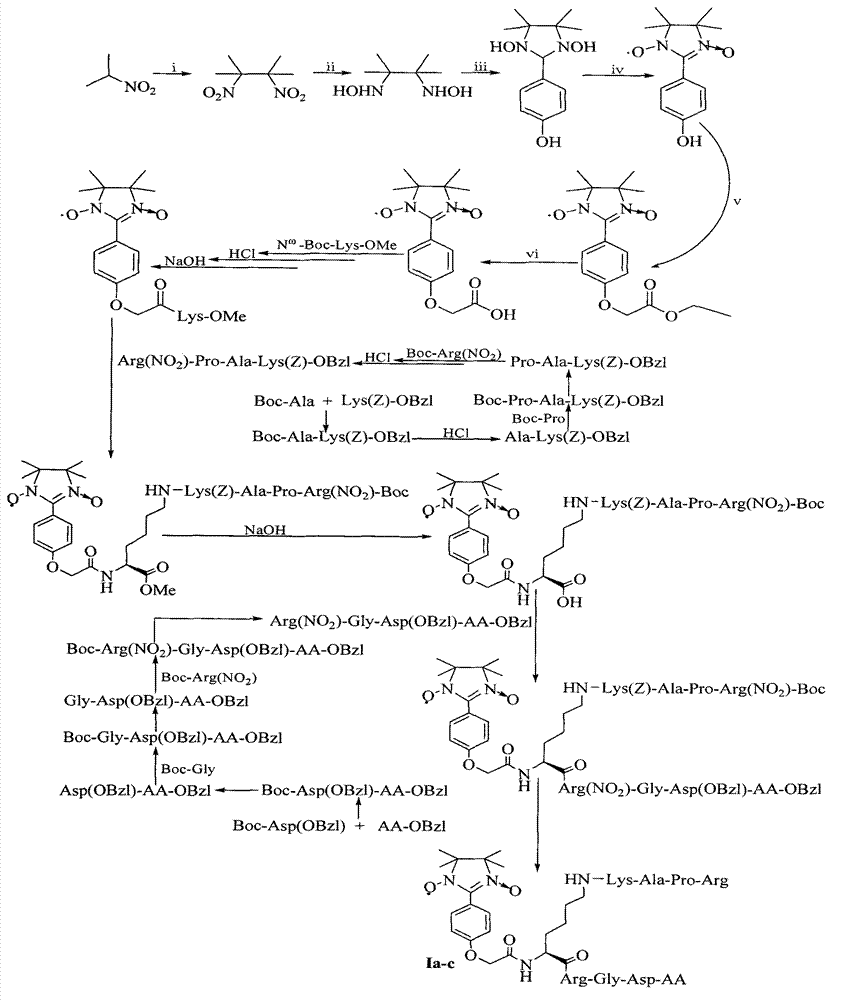
Examples
Embodiment 1
[0036] Example 1 Preparation of 2,3-dimethyl-2,3-dinitrobutane
[0037] 69g (0.78mol) of 2-nitropropane was added to 130ml of NaOH (6N) aqueous solution, and 20ml (0.38mol) of Br 2 , Added dropwise within 1 hour, then added 240ml of ethanol, refluxed at 90°C for 3h, poured the reaction solution into 800ml of ice water while heating, and filtered 55g (81%) of the title compound as colorless flaky crystals, Mp110-112°C.
Embodiment 2
[0038] Example 2 Preparation of 2,3-dimethyl-2,3-dihydroxyaminobutane
[0039] 7g (40mmol) 2,3-dimethyl-2,3-dinitrobutane and 4g NH 4 Cl was suspended in 80ml ethanol aqueous solution (50%), stirred under ice bath, and 16g zinc powder was added within 3h. After the zinc powder was added, the ice bath was removed, and the stirring reaction at room temperature was continued for 3 h, and then the reaction liquid was suction-filtered. The filter cake was washed repeatedly with aqueous ethanol (50%), the filtrate and washings were combined, adjusted to pH = 2 with concentrated hydrochloric acid, and steamed under reduced pressure until it became a slurry. Add an appropriate amount of potassium carbonate, mix well, use a Soxhlet extractor, use chloroform as the extractant, and extract for 6 hours. The extract is concentrated under reduced pressure to a small amount. After adding petroleum ether, 2.60 g (44%) of the title compound is precipitated as colorless crystals . Mp157-159°...
Embodiment 3
[0040] Example 3 Preparation of 1,3-dihydroxy-2-(4'-hydroxyphenyl)-4,4,5,5-tetramethylimidazolidine
[0041] 1.22g (10mmol) of p-hydroxybenzaldehyde and 1.48g (10mmol) of 2,3-dimethyl-2,3-dihydroxyaminobutane were dissolved in 10ml of methanol. After stirring at room temperature for 8h, TLC showed that the raw material point disappeared. Filtration with suction gave 1.29 g (51%) of the title compound as colorless crystals. EI-MS(m / z)252[M] + . 1 H-NMR (DMSO-d 6 )δ (ppm) = 1.03 (s, 6H), 1.05 (s, 6H), 4.39 (s, 1H), 6.70 (d, J = 6.9Hz, 2H), 7.23 (d, J = 6.9Hz, 2H) , 7.63(s, 1H), 7.85(s, 2H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com