Atypical kesterite compositions
A technology of kesterite and composition, which is applied in the field of preparing CZTS film, and can solve the problems of severe toxicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0090]
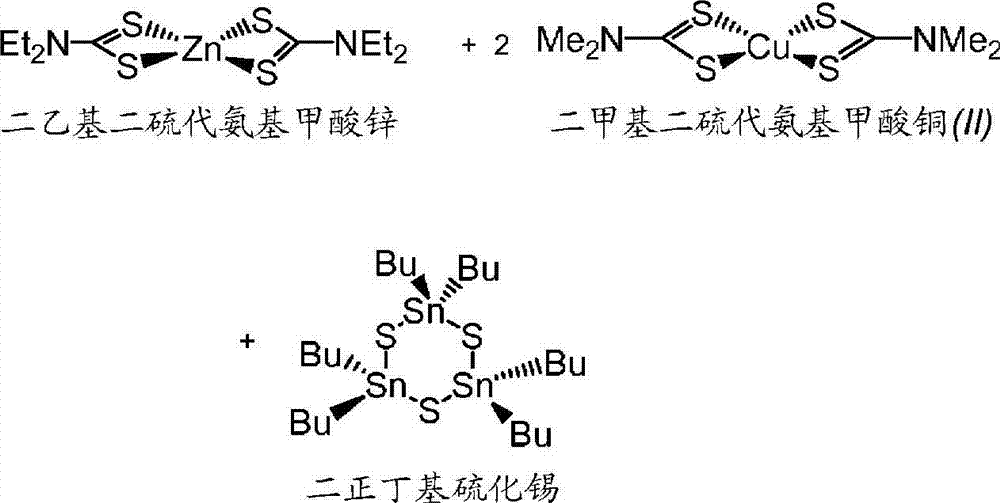
[0091]Zinc diethyldithiocarbamate (0.5919g, 1.635mmol), copper (II) dimethyldithiocarbamate (0.9930g, 3.267mmol) and di-n-butyltin sulfide (0.4506g, 1.701mmol ) into a 40 mL amber vial equipped with a stir bar. 4-tert-butylpyridine (4.4144 g) was added and the resulting mixture was stirred to homogeneity. Then 0.0520 g (1.621 mmol) of elemental sulfur was added. The reaction mixture was stirred at room temperature for ~12 hours, then at the first heating temperature of 105°C for ~40 hours. The reaction mixture was then stirred at a second heating temperature of 190°C for ~8 hours. SLG-coated substrate 1A was prepared by drop coating according to the following method: While maintaining at 105°C under agitation, a small portion of the formulation was drawn into a pipette and spread evenly on a substrate that was also heated to 105°C superior. The coatings on the SLG slides were then dried in a drying oven by increasing the hot plate temperature from 105°C to 170°...
Embodiment 2
[0095]
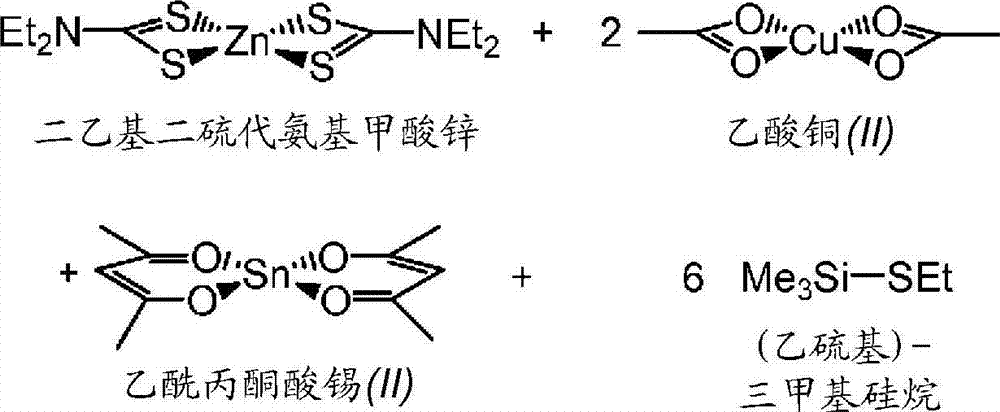
[0096] In a dry box, place zinc diethyldithiocarbamate (0.842 mmol), copper(II) acetate (1.58 mmol), and tin(II) acetylacetonate (0.846 mmol) in a 20 mL vial equipped with a stir bar . 2-Aminopyridine (1.0058 g) was added, followed by 5.05 mmol of (ethylthio)trimethylsilane and 0.814 mmol of sulfur. The reaction mixture was stirred at the first heating temperature of 105°C for ~40 hours. The reaction mixture was then stirred at a second heating temperature of 190°C for ~8 hours. In a similar manner as described above in Example 1, the treated glass slide was coated by drop coating to make Coated Substrate 2A, and dried at 170°C for ~0.5 hours.
[0097] This process was repeated except that 3-aminopyridine was used as solvent to prepare coated substrate 2B. XAS analysis of coated substrates 2A and 2B confirmed the presence of CZTS with low Cu / Zn for 2A and 2B (Cu / Zn=1.06 in kesterite for 2A and 1.06 in kesterite for 2B. Cu / Zn=1.10).
Embodiment 3
[0099]
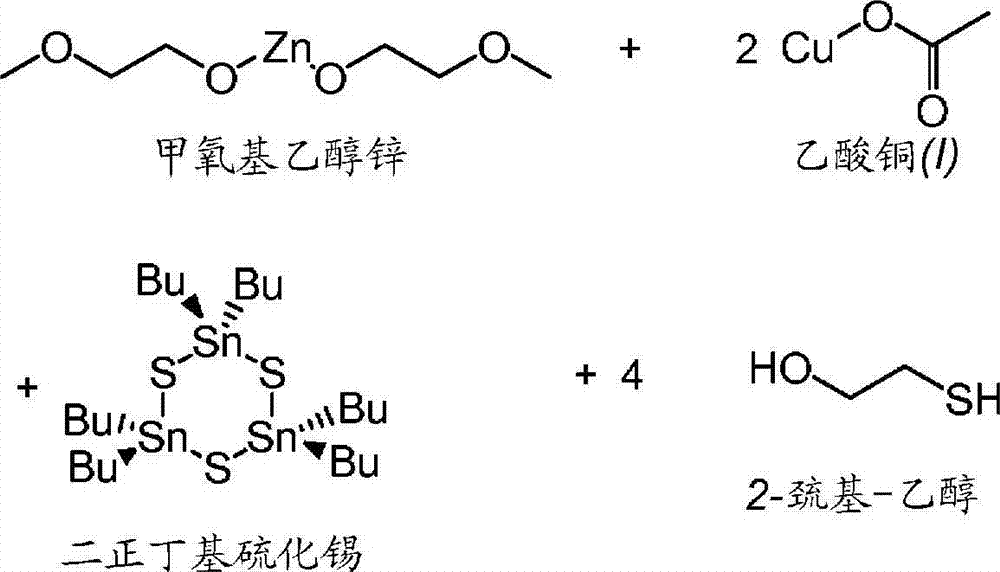
[0100] Zinc methoxyethoxide (1.638 mmol), copper(I) acetate (3.263 mmol), di-n-butyltin sulfide (1.631 mmol) and 2-mercaptoethanol (6.885 mmol) were placed together in a 40 mL amber in vial. 4-tert-butylpyridine (1.857 g) was added, and the resulting mixture was stirred well. Then 1.634 mmol of elemental sulfur were added. The reaction mixture was stirred at room temperature for ~12 hours, then at 105 °C for ~40 hours. The reaction mixture was then stirred at 190°C for ~16 hours. According to the method of Example 1, SLG-coated substrate 3A was prepared by drop coating and dried at 250° C. for 0.5 hours.
[0101] This process was repeated, using 2-aminopyridine as solvent, and the reaction mixture was stirred at a second heating temperature of 170° C. for ~8 hours to produce coated substrate 3B. This process was repeated, using 4-tert-butylpyridine as solvent, and the reaction mixture was stirred at a second heating temperature of 160° C. for ~8 hours to produc...
PUM
| Property | Measurement | Unit |
|---|---|---|
| boiling point | aaaaa | aaaaa |
| energy | aaaaa | aaaaa |
| boiling point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com