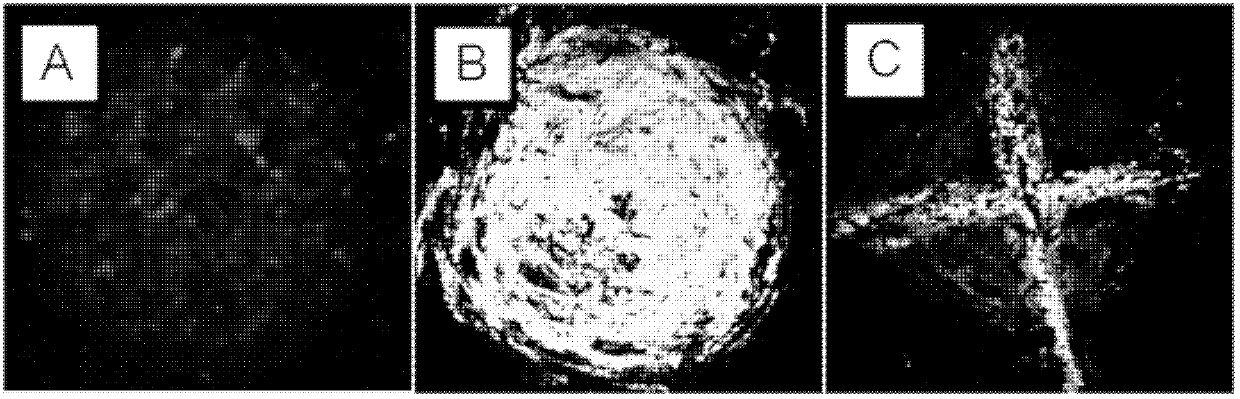
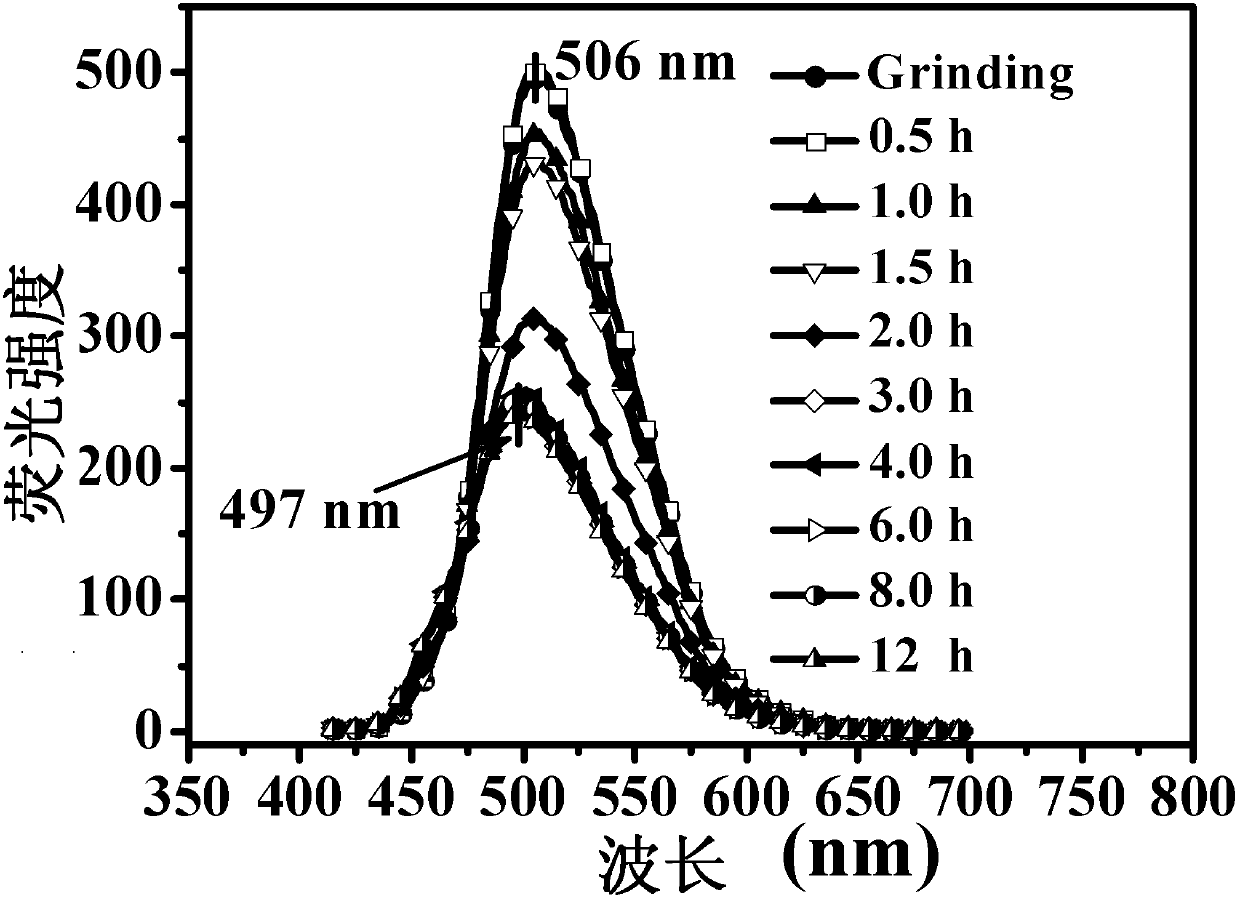
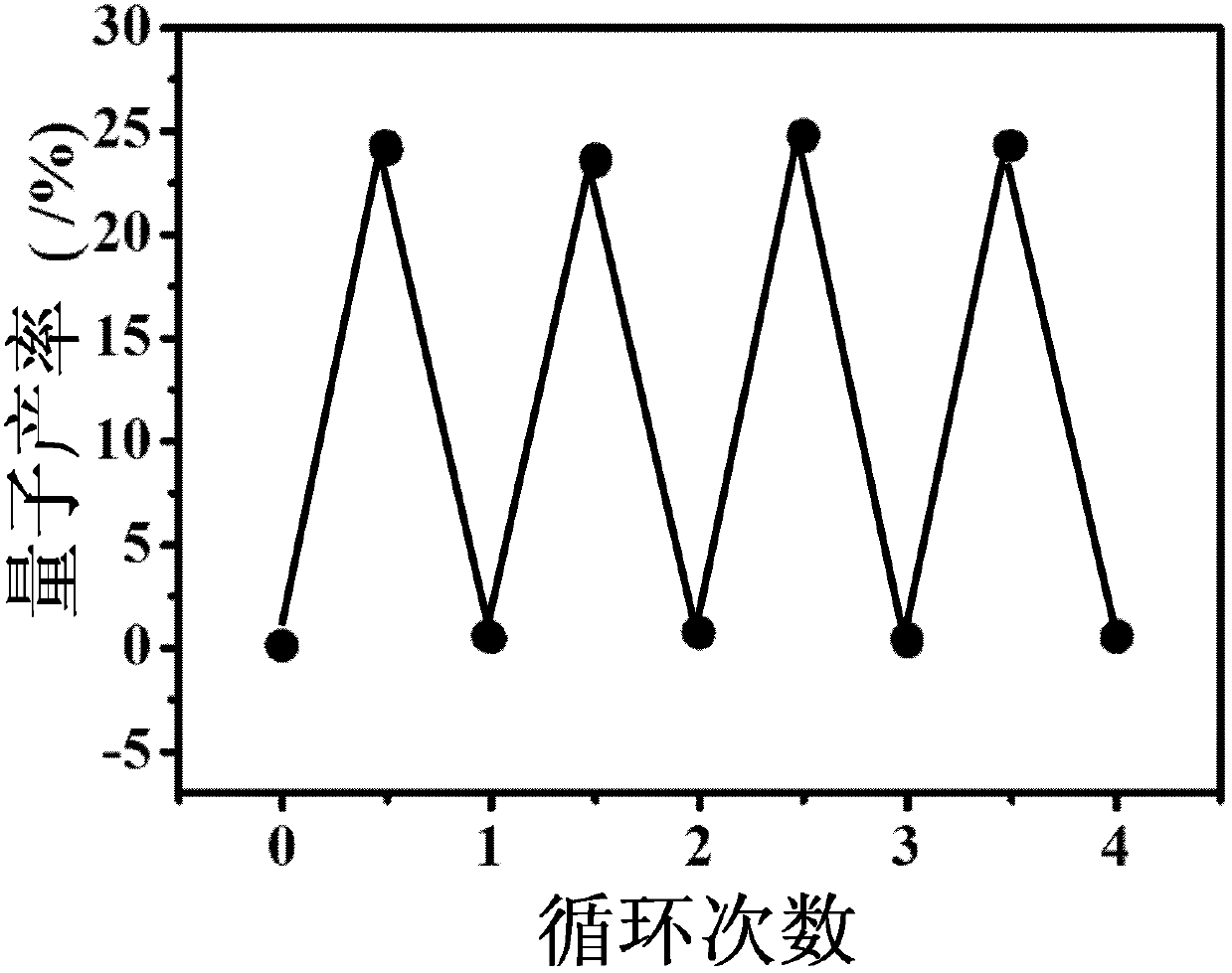
Triphenylamine derivatives, and preparation method and application thereof
A technology of derivatives and triphenylamine, applied in the fields of triphenylamine derivatives and their preparation and application, can solve the problems of limited application of materials and little fluorescence intensity, and achieves the effects of good cyclability, simple synthesis method and convenient preparation of devices
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036]1.63g (12mmol) of p-methoxybenzaldehyde, 1.96g (10mmol) of p-bromonitrile benzyl and 0.06g (1mmol) of sodium methoxide were dissolved in 30ml of chromatographic ethanol. The reaction was stirred at room temperature for 4 h until a large number of solid particles were present to terminate the reaction. Then put it into -20 DEG C refrigerator overnight and then filter, and the filter cake was rinsed with ethanol three times to obtain a white powder, that is, 2.84 g of brominated stilbene nitrile intermediate (IV), with a yield of 90%. The structural confirmation of the substance is characterized as follows: 1 H NMR (500MHz, CDCl 3 ) δ (ppm) 7.78(s, 1H), 7.48(d, J=7.5Hz, 2H), 7.39(d, J=7.5, 2H), 7.34(d, J=7.5, 2H), 6.91(d, J=7.5, 2H), 3.82(s, 1H); 13 C NMR (500MHz, CDCl 3 ); δ161.3, 141.2, 132.7, 131.8, 131.0, 126.1, 119.7, 114.5, 106.6, 56.0; MS (EI): m / e 313.0 (M + ).
Embodiment 2
[0038] Dissolve 1.09 g (8 mmol) of p-methoxybenzaldehyde, 1.96 g (10 mmol) of p-bromonitrile benzyl and 0.03 g (0.5 mmol) of sodium methoxide in 30 ml of chromatographically pure ethanol. The reaction was stirred at room temperature for 3 h until a large number of solid particles were present to terminate the reaction. Then put it into -20 DEG C refrigerator overnight and then filter, and the filter cake was rinsed with ethanol three times to obtain a white powder, that is, 2.19 g of brominated stilbene nitrile intermediate (IV), with a yield of 90%.
Embodiment 3
[0040] Weigh the stilbene nitrile intermediate (IV) 1.57g (5mmol) of the brominated synthesis above, 1.73g (6mmol) of triphenylamine 4-boric acid, and 0.11g (0.1mmol) of tetrakis (triphenylphosphine) palladium. To a solution of 50 mL of toluene / 30 mL of tetrahydrofuran, an aqueous solution of sodium carbonate (2.0 M, 3 mL) was added. Under a nitrogen atmosphere, the temperature was raised to 90° C. for 36 h. The reaction solution was cooled, the solvent was distilled off under reduced pressure, extracted three times with chloroform, the organic phases were combined, washed with saturated sodium carbonate and brine, and finally dried over anhydrous magnesium sulfate. Filtration, the filtrate was concentrated under reduced pressure, and the residue was subjected to thin-layer chromatography with silica gel column chromatography, and the volume ratio of petroleum ether / chloroform was eluted with a mixed solvent of 150 / 1, and the solvent was evaporated under reduced pressure to ob...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com