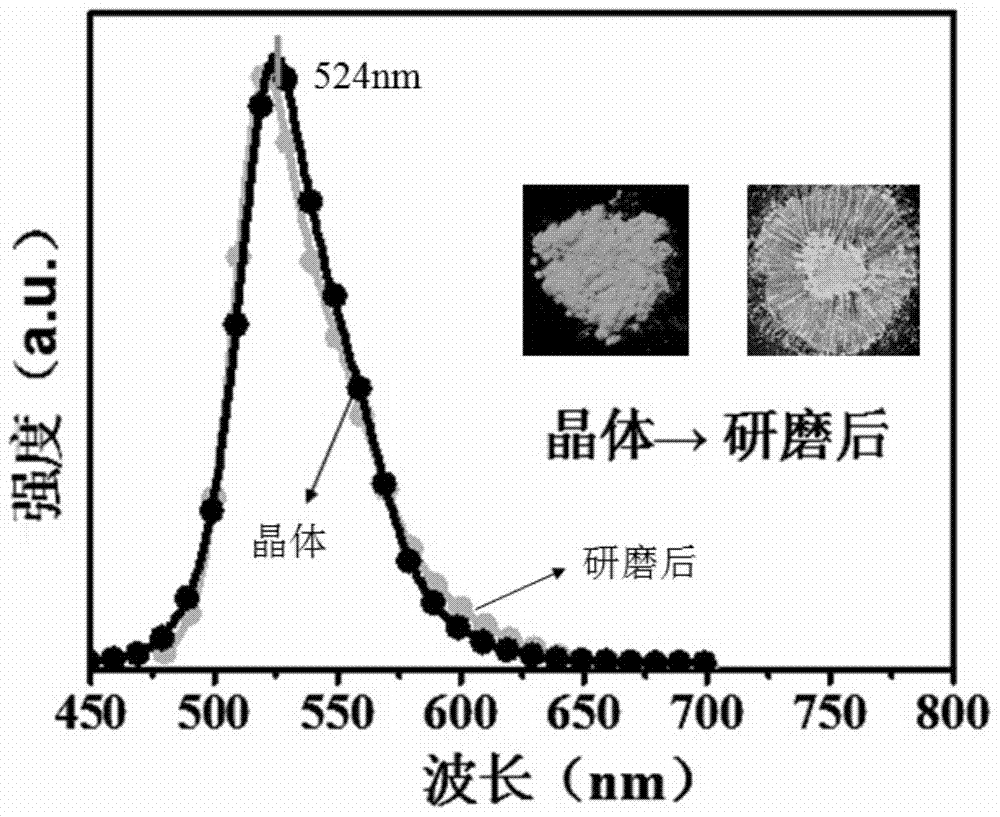
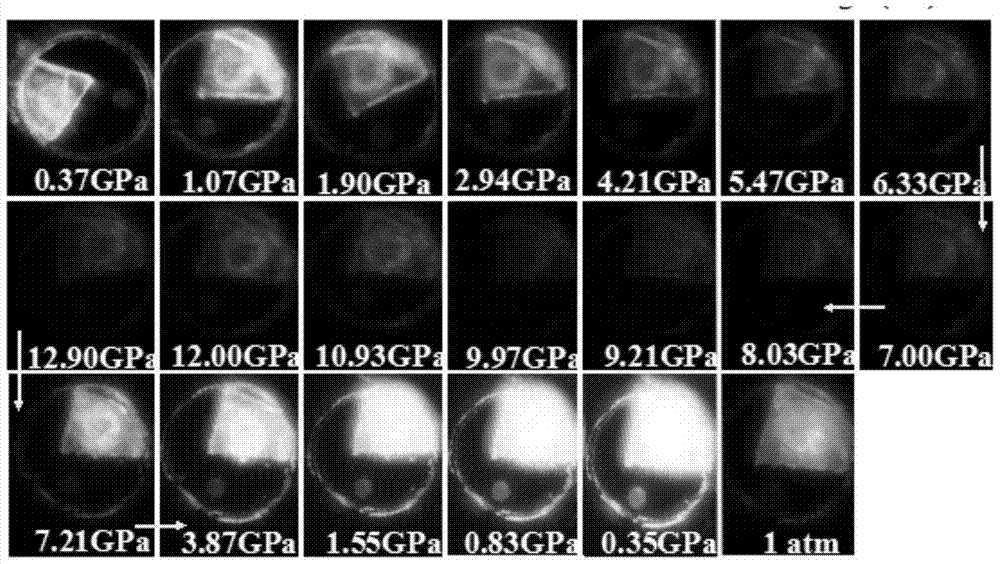
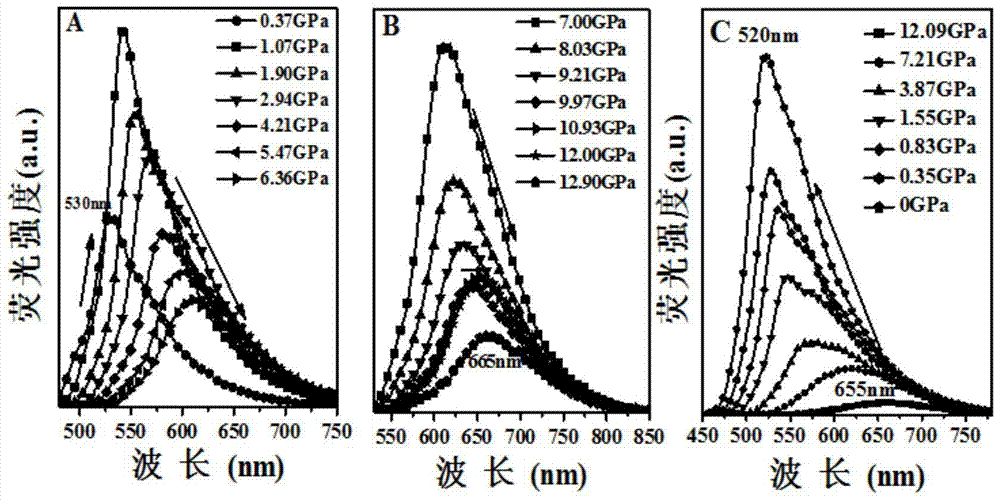
A kind of stilbene nitrile derivative and its preparation method and application
A technology of stilbene nitrile and derivatives, which is applied in the field of stilbene nitrile derivatives and their preparation and application, can solve the problems of limited material application, and achieve the effects of good recyclability, simple synthesis method and high contrast
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] Dissolve 0.92g (5mmol) of p-bromobenzaldehyde (II), 1.73g (6mmol) of triphenylamine (III) 4-boronate, 0.11g (0.1mmol) of tetrakis(triphenylphosphine) palladium in 50mL of toluene / 30mL of tetrahydrofuran and mix To the solution, aqueous sodium carbonate solution (2.0 M, 3 mL) was added. Under a nitrogen atmosphere, the temperature was raised to 90° C. for 36 h. The reaction solution was cooled, and the solvent was evaporated under reduced pressure, then extracted with chloroform (50 mL×3) times, the organic phases were combined, washed with saturated aqueous sodium carbonate solution and saturated brine, and finally dried over anhydrous magnesium sulfate. Filtration, the filtrate was concentrated under reduced pressure, and the residue was separated by silica gel column chromatography. The eluent was a mixed solvent with a volume ratio of petroleum ether / chloroform of 150 / 1. The eluate containing the target compound was collected, and the solvent was evaporated under red...
Embodiment 2
[0047] Dissolve 0.92g (5mmol) of p-bromobenzaldehyde (II), 1.56g (4mmol) of triphenylamine (III) 4-boronate, 0.16g (0.15mmol) of tetrakis(triphenylphosphine) palladium in 40mL of toluene / 25mL of tetrahydrofuran and mix To the solution, aqueous sodium carbonate solution (2.0 M, 3 mL) was added. Under a nitrogen atmosphere, the temperature was raised to 90° C. for 24 h. The reaction solution was cooled, and the solvent was evaporated under reduced pressure, then extracted with chloroform solution (30mL×3), the organic phases were combined, washed with saturated aqueous sodium carbonate solution and saturated brine, and finally dried over anhydrous magnesium sulfate. Filtration, the filtrate was concentrated under reduced pressure, and the residue was separated by silica gel column chromatography. The eluent was a mixed solvent with a volume ratio of chloroform / petroleum ether of 150 / 1. The eluate containing the target compound was collected, and the solvent was evaporated under ...
Embodiment 3
[0049] Weigh 4.19g (12mmol) of the above-synthesized triphenylamine intermediate (IV), 1.33g (10mmol) of 4-methoxybenzonitrile (V) and 0.06g (1mmol) of sodium methoxide and dissolve them in 30ml of chromatographic ethanol. The reaction was stirred at room temperature for 4 h, and the reaction was terminated when a large amount of solid particles were precipitated. Then the reaction system was put into -20 DEG C refrigerator overnight, then filtered, and the filter cake was rinsed with ethanol (50mL * 3), and after natural drying, an orange powder was obtained, i.e. the target product stilbene nitrile derivative (I) 3.82g, The yield is 80%. The structural confirmation of the substance is characterized as follows: 1 H NMR (500MHz, CDCl3): δ7.93(d, J=8.3Hz, 2H); 7.68(t, J=16.2Hz, 2H); 7.64-7.61(m, 2H); 7.52(d, J=8.3 Hz,2H); 7.44(s,1H); 7.29(t,J=7.6Hz,4H); 7.10(dd,J=47.4,5.5Hz,8H);6.99-6.95(m,2H);3.86(s ,3H).MS m / z:478.4
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com