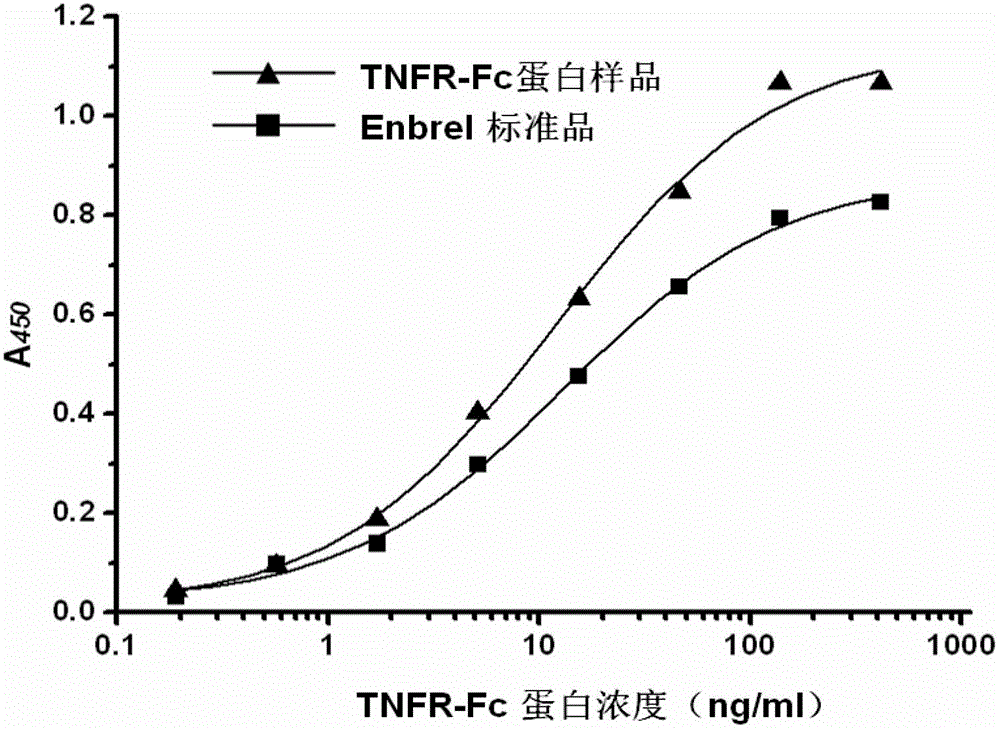
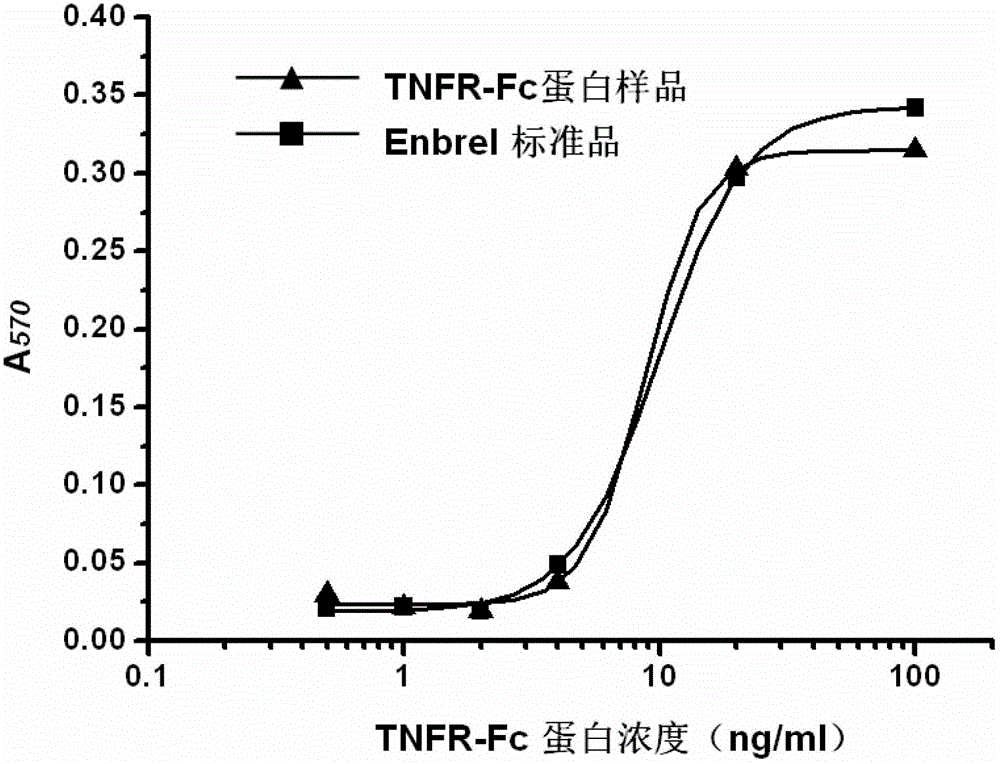
Gene for coding recombinant human TNFR-Fc fusion protein and application of gene
A fusion protein and coding technology, applied in the protein field, can solve the problems of low cost and high price, and achieve the effects of high affinity, simple operation and prolonging cell growth time.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] According to the preference of codons used by CHO mammalian cells, the present invention has known the amino acid sequence of TNFR-FC protein, and designed 4 new gene sequences encoding TNFR-FC protein as follows:
[0037] Sequence 1:
[0038] ATGGCCCCCGTGGCCGTGTGGGCTGCCCTGGCCGTGGGACTGGAACTG TGGGCTGCTGCCCACGCC CTCCCGGCCCAGGTCGCCTTCACCCCGTACGCCCCGGAGCCAGGCTCCACCTGCAGGCTCAGAGAGTACTACGACCAGACCGCCCAGATGTGCTGCTCCAAGTGCTCTCCAGGCCAGCATGCCAAGGTCTTCTGCACCAAGACCTCGGACACCGTCTGCGACTCGTGCGAGGACTCGACCTACACCCAGCTCTGGAACTGGGTCCCGGAGTGCCTCTCGTGTGGCTCGAGGTGCTCTTCGGACCAGGTGGAGACCCAGGCCTGCACCAGAGAGCAGAACAGGATCTGCACCTGCAGACCGGGCTGGTACTGCGCCCTCTCGAAGCAGGAAGGCTGCAGGCTGTGCGCCCCACTCAGGAAGTGCAGGCCGGGCTTCGGCGTCGCCAGACCGGGCACCGAGACCTCCGACGTCGTCTGCAAGCCCTGCGCCCCAGGCACCTTCTCGAACACCACCTCGTCCACCGACATCTGCAGGCCCCACCAGATCTGCAACGTCGTCGCTATCCCGGGCAATGCCTCGATGGACGCCGTCTGCACCTCGACCTCGCCGACCAGATCGATGGCCCCAGGCGCCGTCCACCTGCCGCAGCCGGTCTCCACCAGGTCGCAGCACACCCAGCCCACCCCAGAGCCTAGCACCGCCCCCTCTACCAGCTTCCTGCTGCCCATGG...
Embodiment 2
[0054] Transfection and expression of fusion genes in Chinese hamster ovary cells (CHO cells, Cell Resource Center, Shanghai Institute of Biological Sciences, Chinese Academy of Sciences).
[0055] The clones pIRESneo3-TNFR-Fc-1, pIRESneo3-TNFR-Fc-2, pIRESneo3-TNFR-Fc-3 and pIRESneo3-TNFR-Fc-4 were respectively inoculated in liquid LB medium containing 100 μg / ml ampicillin, 37 Cultivate overnight at ℃, and extract plasmid DNA with μltrapure Plasmid Purification Kit (QIAGEN).
[0056] CHO cells were transfected with liposomes, and the transfection kit was purchased from Invitrogen. During transfection, take 100 μg of the purified pIRESneo3-TNFR-Fc-1, pIRESneo3-TNFR-Fc-2, pIRESneo3-TNFR-Fc-3 and pIRESneo3-TNFR-Fc-4 plasmids as DNA samples to transfect CHO cells , the transfection procedure was carried out according to the manufacturer's instructions.
[0057] After transfection, the CHO cells were continuously treated with puromycin (PM) for 3 months, the concentration was fro...
Embodiment 3
[0072] Fermentation culture in 55L disposable bioreactor
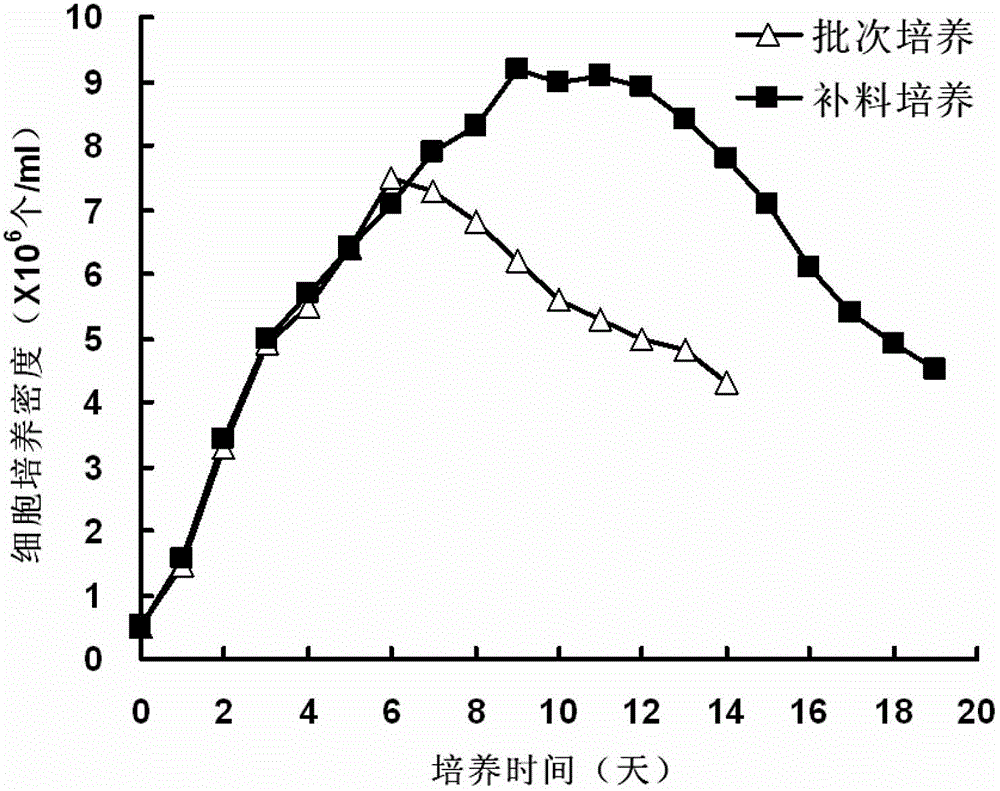
[0073] (1) Take the recombinant CHO cell line constructed and screened (the clone No. 25 of the recombinant TNFR-Fc-2 gene), and use about 5×10 5 The cells / ml were inoculated in proCHO5 medium containing 4mM glutamine, 0.1mM hypoxanthine and 0.016mM thymine, using 50ml Tubespin, the culture volume was 10ml, the culture temperature was 37°C, and the rotation speed was 180rpm. In the process of cell subculture and expansion, the culture is enlarged step by step. After cell expansion in 250ml, 500ml, 1000ml and 5000ml shake flasks, when the cell density reaches 4×10 6 When cells / ml, the transfer adopts 55L disposable bioreactor bags for batch culture, and the culture volume is 32L. During fermentation and growth, according to the growth law of recombinant CHO cells, cultivate them according to conventional methods, initially adjust the speed at 90rpm / min, keep the temperature at 37°C, adjust the air flow at 10L / h, contro...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com