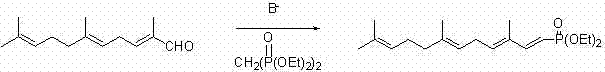
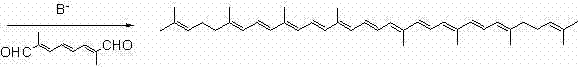
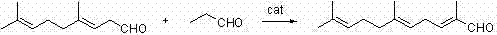Preparation method of 2,6,10-trimethyl-2,5,9-undecane triene-1-aldehyde
A trimethyl, 10-technology, applied in the field of synthesis of lycopene intermediates, can solve the problems of difficult synthesis, difficult industrial production, and difficult sources, and achieve the effects of low cost, easy raw materials, and simple process routes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Example 1: Preparation of 2,6,10-trimethyl-2,5,9-undecatrien-1-al (Formula 2).
[0026] Add 15 grams of L-proline (0.13mol) and 50 milliliters of water into a 500-mL four-neck flask, stir mechanically at 45°C, add dropwise 116 grams of propionaldehyde (2.0 mol), 166 grams of 4,8-dimethyl The mixture of 3,7-nonadien-1-al (6) (1.0mol) and 150 ml of toluene was added dropwise in about 30 minutes, and after stirring for 10 minutes, the reaction solution was cooled to room temperature and left to stand. The layers were separated, the water layer was extracted with 20 ml of toluene (the water layer was the catalyst layer and could be recycled), the combined organic layers were washed with saturated brine, dried, the solvent was recovered, and the target product 2 with a GC content of 96.1% was obtained by rectification under reduced pressure. 170.6 g of 6,10-trimethyl-2,5,9-undecatrien-1-al, yield 79.6%.
[0027] Product structure confirmation:
[0028] 1 HNMR (δppm, 400MH...
Embodiment 2
[0033] Example 2: Preparation of 2,6,10-trimethyl-2,5,9-undecatrien-1-al (Formula 2).
[0034] Add 30g of acetic acid (0.5mol) and 30ml of water into a 500mL four-neck flask, slowly add 56g of 40% dimethylamine aqueous solution (0.5mol), and add 87g of propionaldehyde dropwise at 65°C under mechanical stirring (2.5mol), 166 grams of 4,8-dimethyl-3,7-nonadien-1-al (6) (1.0mol) and 150 ml of dichloroethane, the mixture was added dropwise in about 30 minutes , continue stirring and reacting for 10 minutes, cool the cooled reaction solution to room temperature and let stand to separate layers. The water layer is extracted with 20 ml of dichloroethane (the water layer is the catalyst layer, which can be recovered and used mechanically). The combined organic layers are washed with saturated brine and dried. , recovered the solvent, and rectified under reduced pressure to obtain 178.2 g of the target product 2,6,10-trimethyl-2,5,9-undecatrien-1-al with a GC content of 97.4%, with a y...
Embodiment 3
[0036] Example 3: Preparation of 2,6,10-trimethyl-2,5,9-undecatrien-1-al (2).
[0037]Add 40 grams of acetic acid (0.25mol) and 20 milliliters of water into a 500-mL four-neck flask, slowly add 28 grams of 40% dimethylamine aqueous solution (0.25 mol), and add 145 grams of propionaldehyde dropwise at 80°C under mechanical stirring (2.5mol), 166 grams of 4,8-dimethyl-3,7-nonadien-1-al (6) (1.0mol) and 150 ml of isobutyl acetate, the mixture was added dropwise in about 30 minutes , continue to stir and react for 10 minutes, cool the reaction solution to room temperature and let stand to separate layers, extract the water layer with 20 ml of isobutyl acetate (the water layer is the catalyst layer, which can be recycled and applied), the combined organic layers are washed with saturated brine, and dried , recovered the solvent, and rectified under reduced pressure to obtain 172.2 g of the target product 2,6,10-trimethyl-2,5,9-undecatrien-1-al with a GC content of 96.4%, with a yie...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com