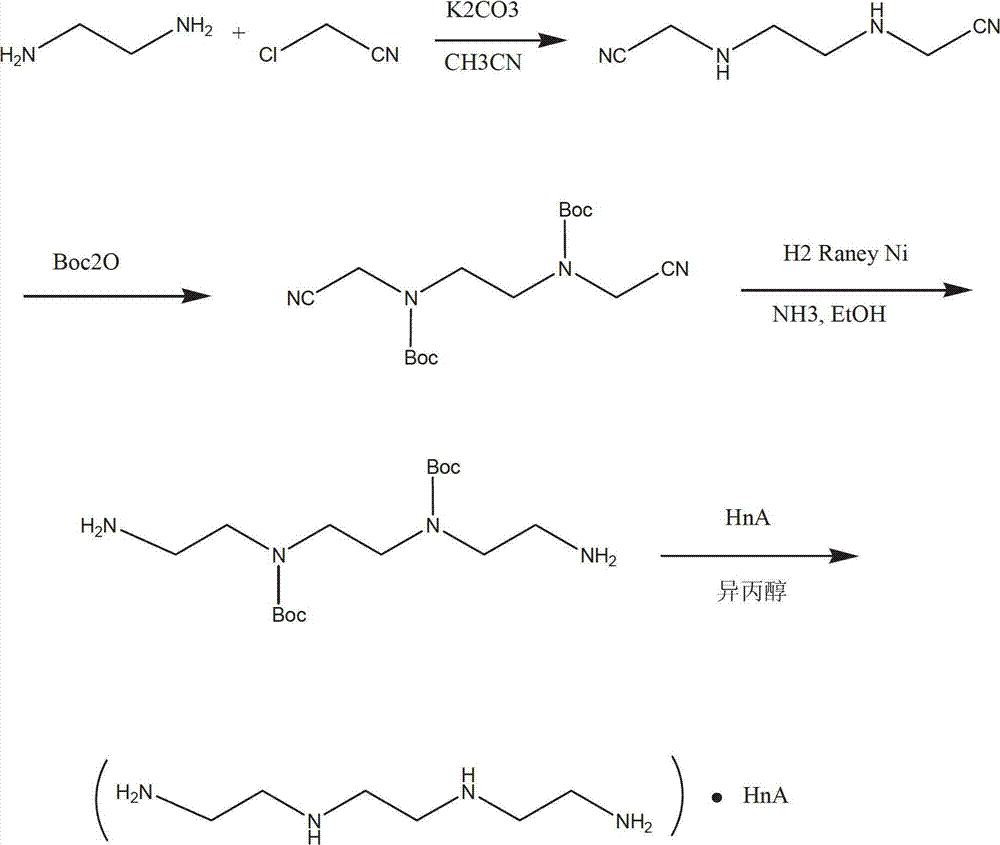
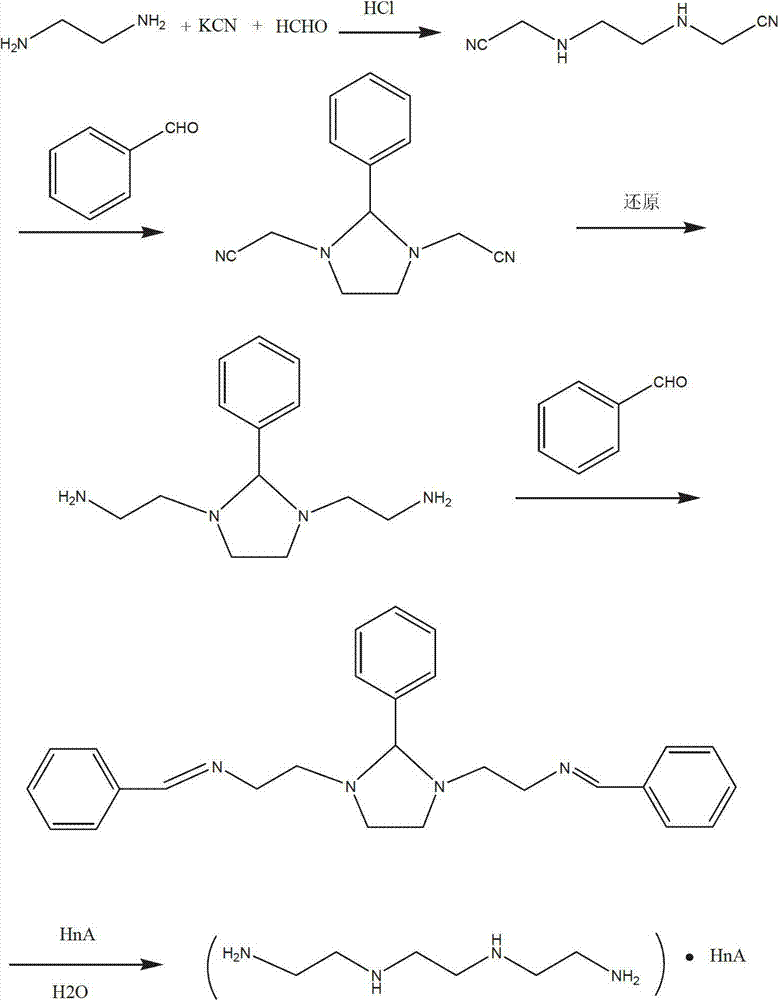
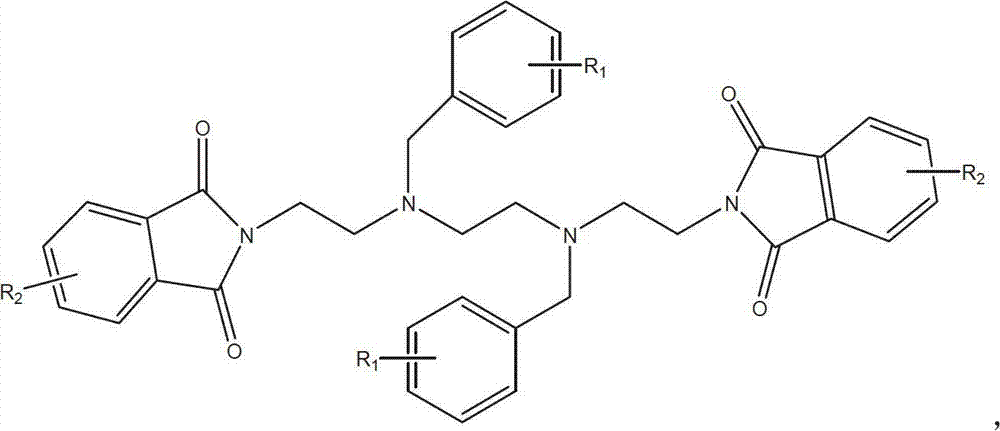
Synthetic process of hydrochloric acid trientine
A hydrochloric acid and new process technology, applied in the preparation of amino compounds, the preparation of organic compounds, organic chemistry, etc., can solve the problems of difficult control of the conditions for dihydrochloride formation, and achieve the effects of easy conditions, easy operation and little pollution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] 1) Preparation of Intermediate B:
[0037] The molar ratio of starting material A: absolute ethanol: 80% hydrazine hydrate is 1:275:7.8.
[0038] Preparation method: Add 276g of starting material A: 2,2'-(2,2'-(ethane-1,2-diyl -Bisbenzyl-bis(ethane-2,1-diyl))diisoindole-1,3-dione, 7.5L absolute ethanol, 230mL80% hydrazine hydrate, heated to 60-70°C under stirring, React for 18 hours, stir and cool to room temperature, continue to stir for 2 hours, filter with suction, wash the filter residue with absolute ethanol, concentrate the mother liquor on a rotary evaporator until there is no distillate, and reclaim ethanol. Add appropriate amount of anhydrous to the solid in the bottle Ethanol, stirred, suction filtered, and the filter residue was washed with a small amount of absolute ethanol. The combined filtrate was concentrated to dryness under reduced pressure on a rotary evaporator to obtain a yellow oil, which was Intermediate B: N1, N1'-(B alkane-1,2-diyl)bis(N1-benz...
Embodiment 2
[0051] 1) Preparation of Intermediate B:
[0052] The molar ratio of starting material A: absolute ethanol: 60% hydrazine hydrate is 1:270:10.
[0053]Preparation method: Add 276g of starting material A: 2,2'-(2,2'-(ethane-1,2-diyl -Bisbenzyl-bis(ethane-2,1-diyl))diisoindole-1,3-dione, 7.0L absolute ethanol, 390mL60% hydrazine hydrate, heated to 60-70°C under stirring, React for 20 hours, stir and cool to room temperature, continue to stir for 2 hours, filter with suction, wash the filter residue with absolute ethanol, concentrate the mother liquor on a rotary evaporator until there is no distillate, and recover ethanol. Add appropriate amount of anhydrous to the solid in the bottle Ethanol, stirred, suction filtered, and the filter residue was washed with a small amount of absolute ethanol. The combined filtrate was concentrated to dryness under reduced pressure on a rotary evaporator to obtain a yellow oil, which was Intermediate B: N1, N1'-(B alk-1,2-yl)bis(N1-benzylethan...
Embodiment 3
[0062] 1) Preparation of Intermediate B:
[0063] The molar ratio of starting material A: absolute ethanol: 70% hydrazine hydrate is 1:300:7.
[0064] Preparation method: Add 276g of starting material A: 2,2'-(2,2'-(ethane-1,2-diyl -Bisbenzyl-bis(ethane-2,1-diyl))diisoindole-1,3-dione, 8.3L absolute ethanol, 235mL70% hydrazine hydrate, heated to 60-70°C under stirring, React for 15 hours, stir and cool to room temperature, continue to stir for 2 hours, filter with suction, wash the filter residue with absolute ethanol, concentrate the mother liquor on a rotary evaporator until there is no distillate, and reclaim ethanol. Add appropriate amount of anhydrous to the solid in the bottle Ethanol, stirred, suction filtered, and the filter residue was washed with a small amount of absolute ethanol. The combined filtrates were concentrated to dryness on a rotary evaporator under reduced pressure to obtain a yellow oil, which was Intermediate B.
[0065] 2) Preparation of Intermediat...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com