Preparation method of crystal form I of clopidogrel hydrogen sulfate
A technology of clopidogrel hydrogen sulfate and clopidogrel free base, which is applied in the field of preparation of clopidogrel hydrogen sulfate crystal form I, can solve the problems of high production cost, simple and reliable process, and low solvent recovery rate, and achieve The effect of small health hazards to personnel, simple and easy process, and high solvent recovery rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
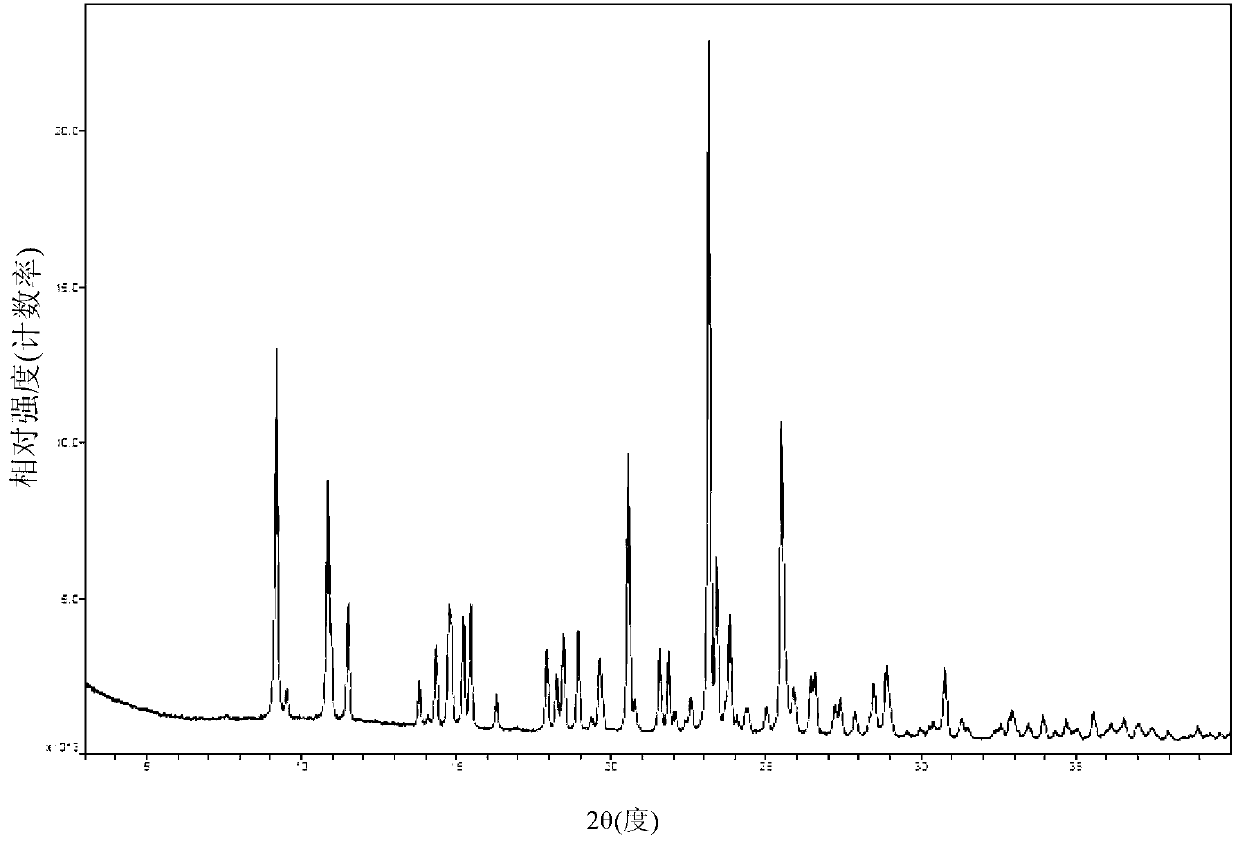
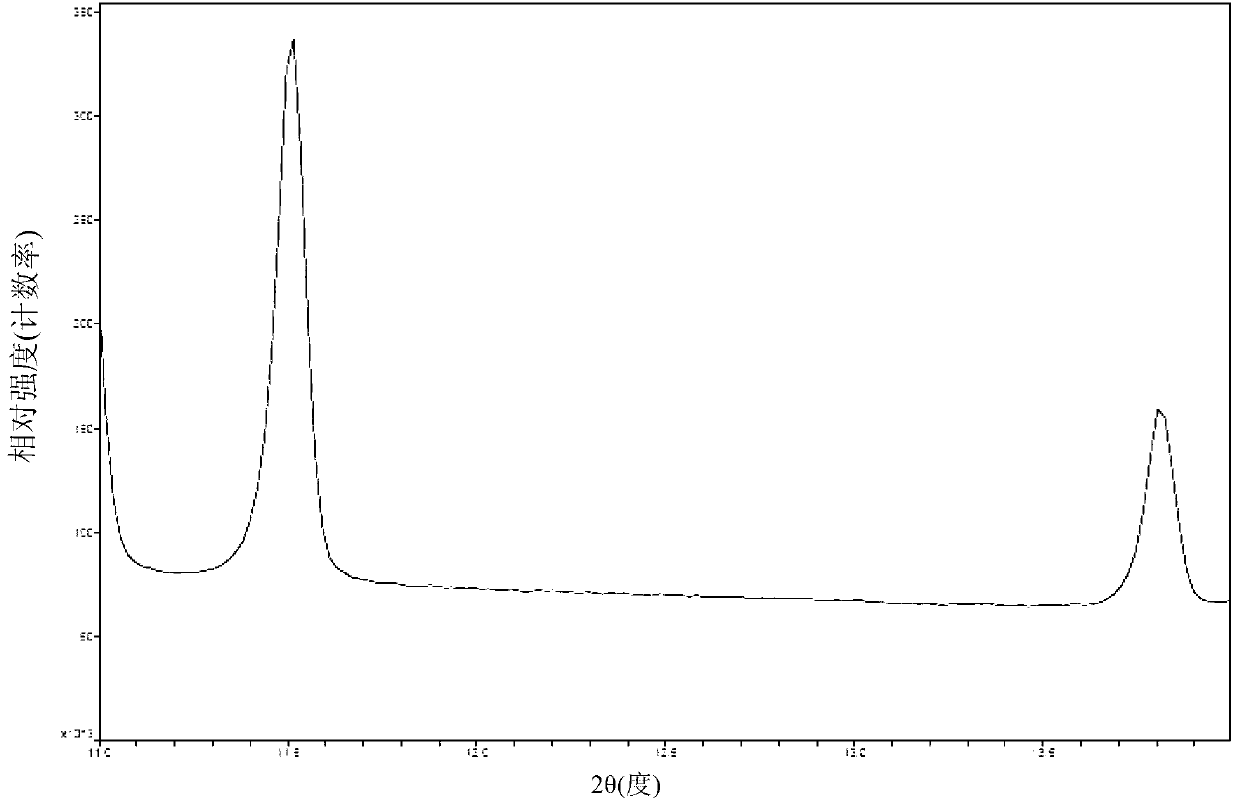
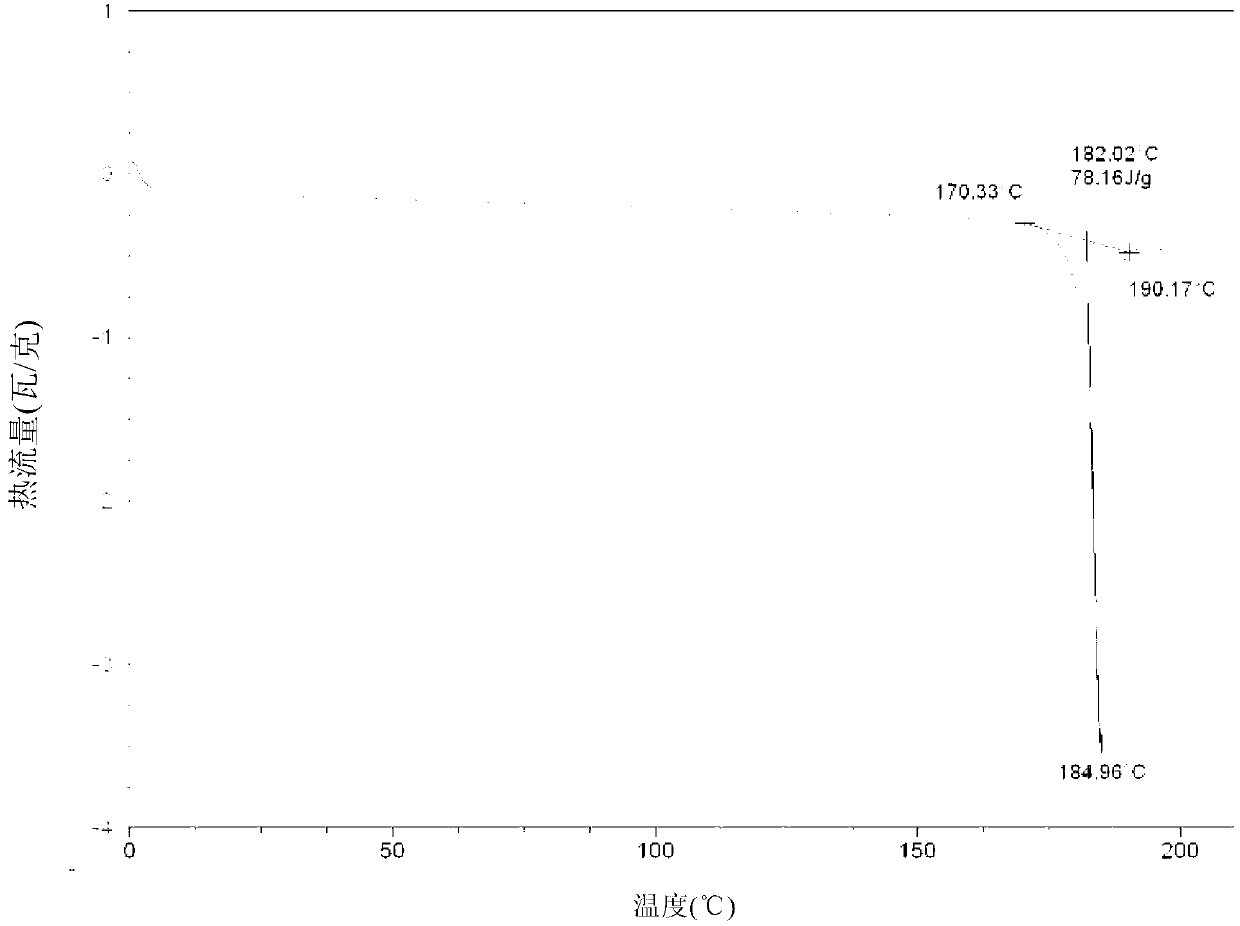
[0057] Add 32.1g (0.10 mol) of clopidogrel free base and 214g of 2-methyltetrahydrofuran into a 1000mL four-neck flask, stir and dissolve at room temperature, cool the system to -10°C, add 9.5g of 98% concentrated sulfuric acid (0.095 mol) dropwise, and then The system was heated up to 10°C, kept stirring and crystallized for 20 hours, filtered under nitrogen pressure, and the filter cake was vacuum-dried at room temperature for 3 hours, then heated to 45°C and vacuum-dried for 10 hours to obtain 39.3g of clopidogrel bisulfate crystal form I , the molar yield is 93.8%, the HPLC content is 99.6%, and the HPLC content of all single heterogeneous compounds is less than 0.1%.
Embodiment 2
[0059] Add 32.1g (0.10 mol) of clopidogrel free base and 270g of 2-methyltetrahydrofuran into a 500mL four-neck flask, stir and dissolve, control the temperature of the system at -5°C, add 9.8g of 98% concentrated sulfuric acid (0.098 mol) dropwise, and the addition is complete Afterwards, the system was heated up to 20°C, kept stirring and crystallized for 12 hours, filtered under nitrogen pressure, washed the filter cake with 100mL 2-methyltetrahydrofuran, filtered under nitrogen pressure, and dried under vacuum at 40°C for 10 hours to obtain 39.9g of clopidogrel sulfate Hydrogen salt crystal form I, molar yield 95.0%, HPLC content 99.8%, all single hetero HPLC content less than 0.1%.
Embodiment 3
[0061] Add 32.1g (0.10mol) of clopidogrel free base and 321g of 2-methyltetrahydrofuran into a 500mL four-neck flask, stir and dissolve, control the temperature of the system at 0°C, add 9.8g of 98% concentrated sulfuric acid (0.098mol) dropwise, after the addition is complete The system was heated up to 25°C, kept stirring and crystallized for 10 hours, filtered under nitrogen pressure, and the filter cake was vacuum-dried at 40°C for 10 hours to obtain 40.6g of clopidogrel bisulfate crystal form I, with a molar yield of 96.7% and an HPLC content of 99.8 %, all simple HPLC content is less than 0.1%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com