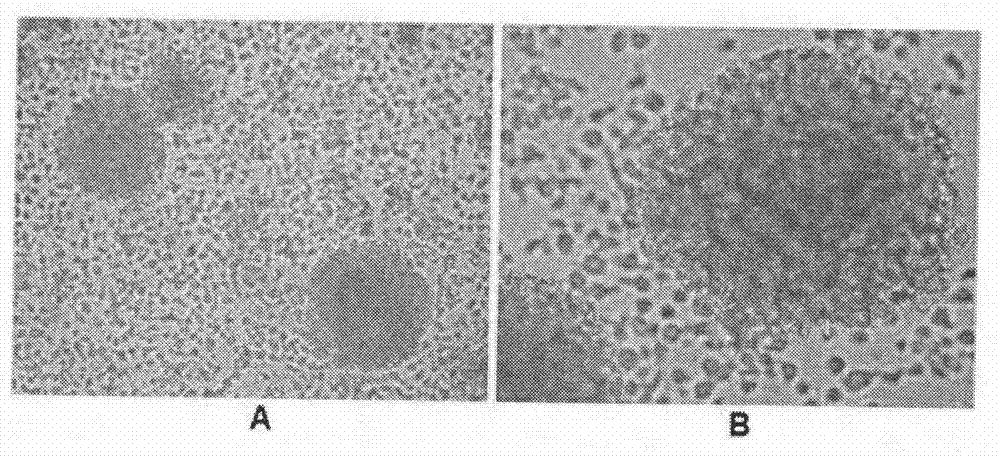
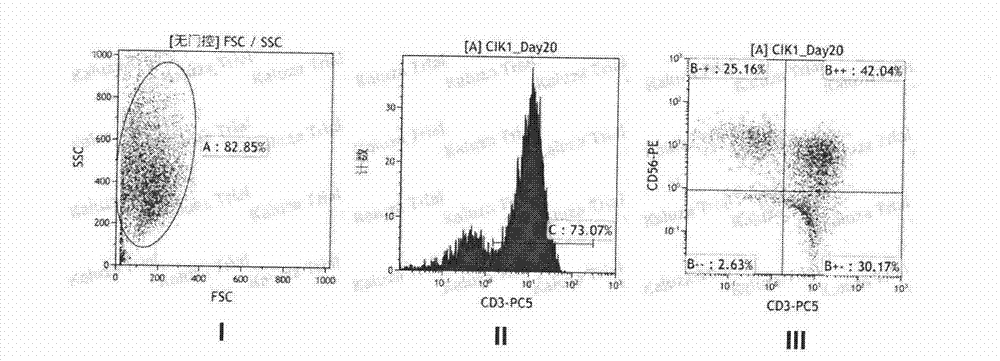
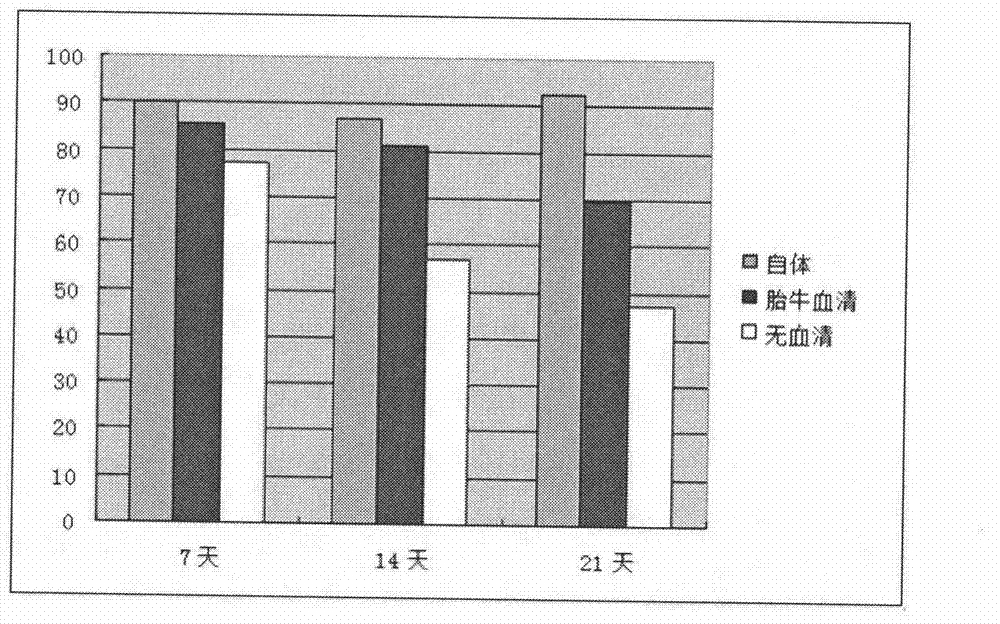
Method for preparing CIK cell by using heparin anticoagulant plasma
A plasma and cell technology, applied in the biological field, can solve the problems of limited popularization and application, high price of serum-free medium, etc., and achieve the effects of improved biological activity and stable activity.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Embodiment 1: The method of using the heparin anticoagulated plasma of the patients after cardiac cancer operation to cultivate autologous lymphocytes, the specific content is as follows:
[0031] 1. Clinical data
[0032] Case 1, male, 56 years old, 2 months after surgery for cardiac cancer, was confirmed by pathological examination after surgery.
[0033] 2. Main materials
[0034] Hyclone RPMI-1640 was purchased from Thermo Fisher Biochemical Products (Beijing) Co., Ltd.; recombinant human interleukin-2 (Recombinant human interleukin-2, rhIL-2) for injection was purchased from Beijing Sihuan Biopharmaceutical Co., Ltd.; Recombinant human interleukin-1 alpha (rhIL-1α) was purchased from PeproTech, USA; Recombinant human interferon γ (rhIFN-γ) for injection was purchased from Shanghai Kaimao Biomedical Co., Ltd. Company; anti-human T cell CD3 mouse monoclonal antibody (Monoclonal antibody CD3, CD3McAb) for injection was purchased from Wuhan Institute of Biological Pr...
Embodiment 2
[0042] Embodiment 2: The method of using the heparin anticoagulated plasma of the patient after left breast cancer operation to cultivate autologous lymphocytes, the specific content is as follows:
[0043] 1. Clinical data
[0044] Case 2, female, 54 years old, left breast cancer after 1 year, was confirmed by pathological examination after surgery.
[0045] 2. Main materials
[0046] Hyclone RPMI-1640 was purchased from Thermo Fisher Biochemical Products (Beijing) Co., Ltd.; recombinant human interleukin-2 (Recombinant human interleukin-2, rhIL-2) for injection was purchased from Beijing Sihuan Biopharmaceutical Co., Ltd.; Recombinant human interleukin-1 alpha, rhIL-1α) was purchased from PeproTech, USA; recombinant human interferon γ for injection (Recombinat human interferon γ, rhIFN-γ) was purchased from Shanghai Kaimao Biopharmaceutical Co., Ltd. ; Anti-human T cell CD3 mouse monoclonal antibody (Monoclonal antibody CD3, CD3McAb) for injection was purchased from Wuhan ...
Embodiment 3
[0060] Embodiment 3: The method of using heparin anticoagulated plasma of patients with gastric cancer to cultivate autologous lymphocytes, the specific content is as follows:
[0061] 1. Clinical data
[0062] Case 3, male, 50 years old, gastric cancer patient.
[0063] 2. Main materials
[0064] Hyclone RPMI-1640 was purchased from Thermo Fisher Biochemicals (Beijing) Co., Ltd. Recombinant human interleukin-2 (rhIL-2) for injection was purchased from Beijing Sihuan Biopharmaceutical Co., Ltd. Recombinant human interleukin-1 alpha (rhIL-1 alpha) was purchased from PeproTech, USA. Recombinant human interferon γ for injection (Recombinat human interferon γ, rhIFN-γ) was purchased from Shanghai Kaimao Biopharmaceutical Co., Ltd. Anti-human T cell CD3 mouse monoclonal antibody (Monoclonal antibody CD3, CD3McAb) for injection was purchased from Wuhan Institute of Biological Products, and human lymphocyte separation liquid was purchased from Beijing Soleibao Technology Co., Ltd...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com