Covalent organic frameworks and methods of making same
A technology of covalent organic framework and crystalline state, applied in the direction of organic chemistry, chemical instruments and methods, and other chemical processes, can solve the problem of no COF
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
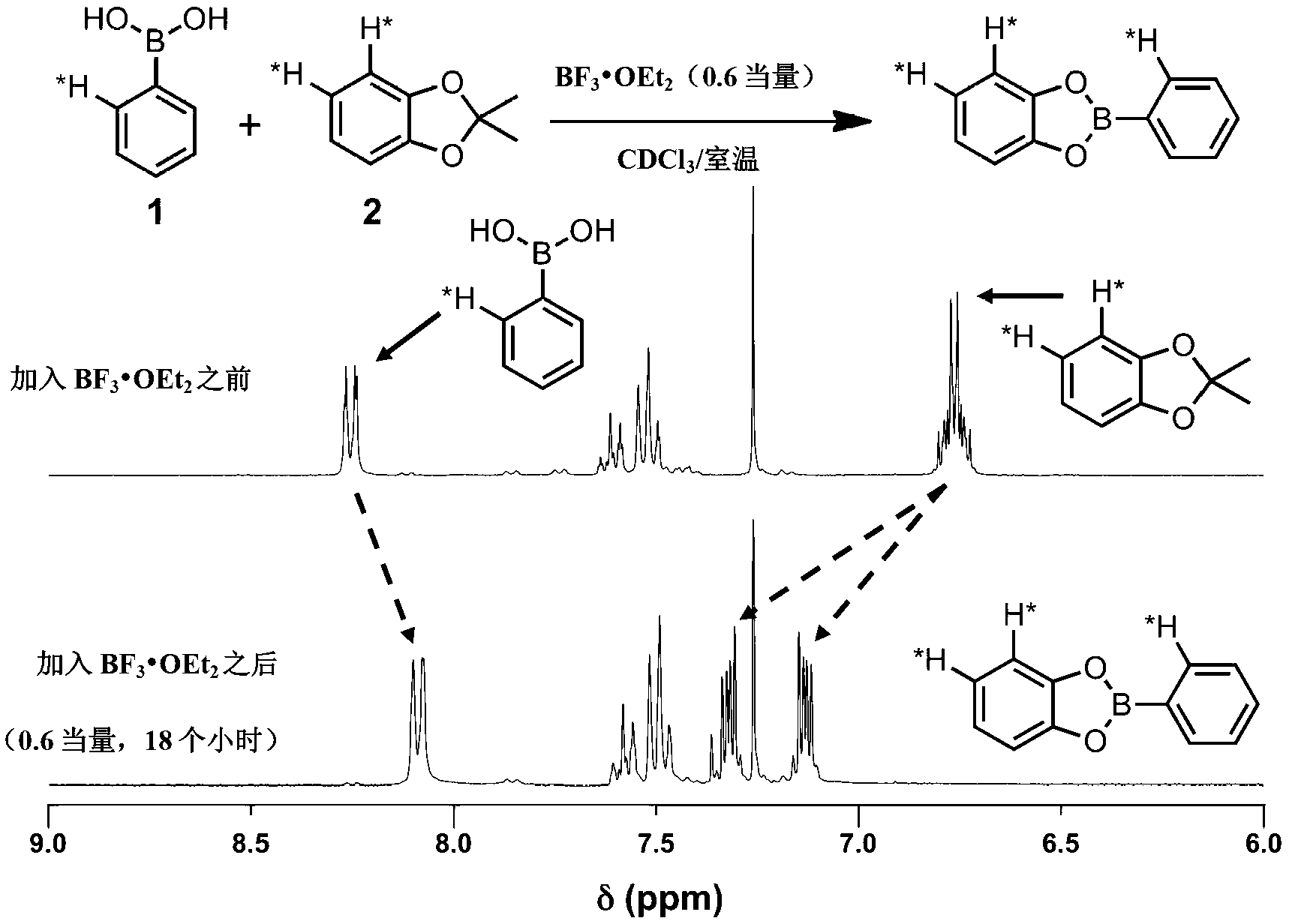
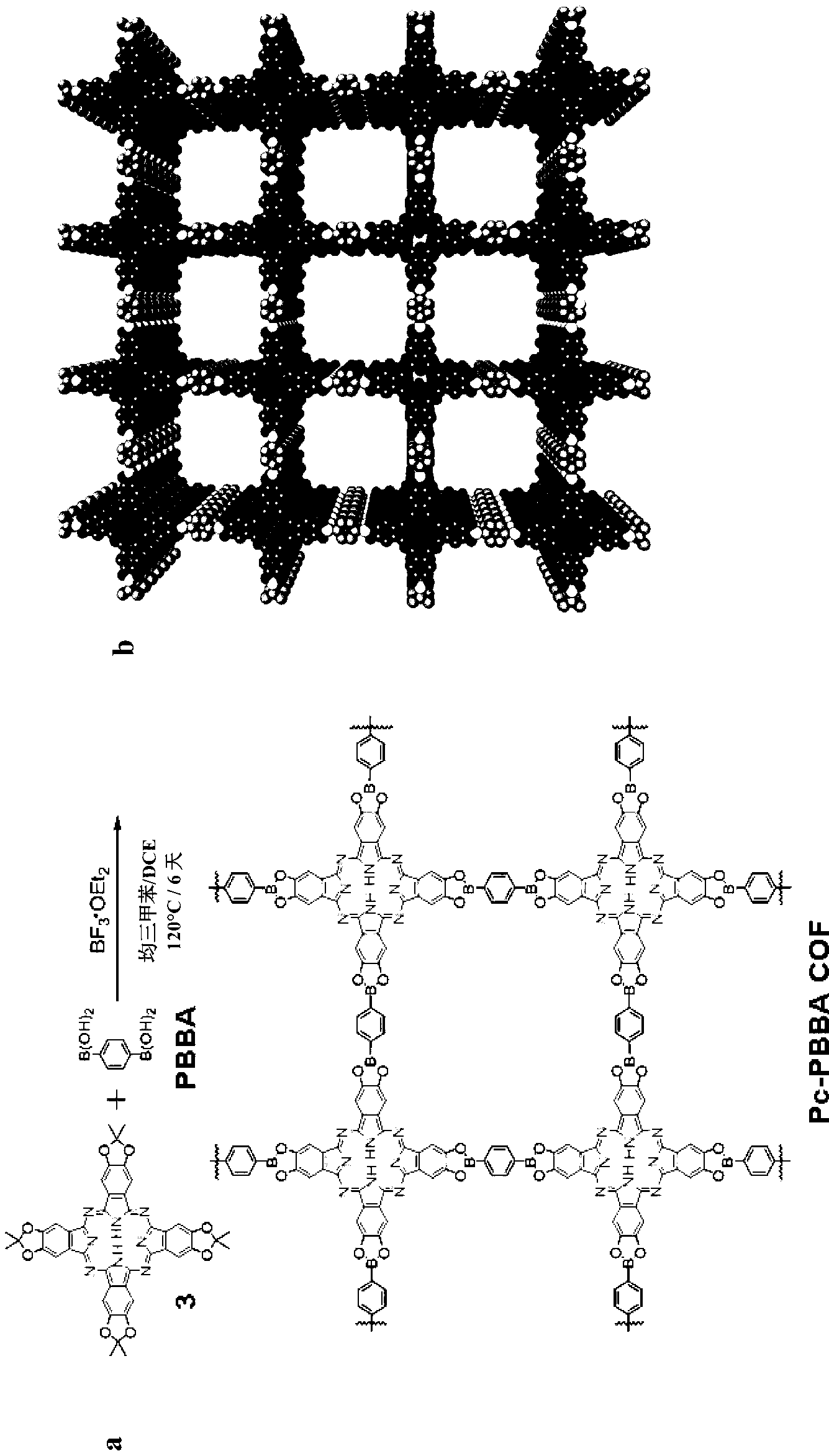
[0090] This example describes a general method for the synthesis of boronic ester-linked COFs that avoids the direct use of insoluble and unstable multifunctional catechol reactants. Using the described method, two-dimensional networks of coplanar stacked phthalocyanines (Pcs), which are strongly absorbing chromophores, have been prepared in bulk heterojunction / dye-sensitized solar cells, as well as for many other applications . The phthalocyanine COFs form overlapping two-dimensional square lattices as determined by powder X-ray diffraction, surface area analysis, and ultraviolet / visible / near-infrared and fluorescence spectroscopy. The materials can be used to form COF-based bulk heterojunctions characterized by structurally precise and high surface area interfaces between complementary organic semiconductors.
[0091] The direct formation of boronate esters from protected catechols provides an attractive option for the synthesis of COFs, since the protecting group can reduc...
Embodiment 2
[0146] Example of COF
[0147] The structure of the COF in this embodiment is as follows Figure 32 shown.
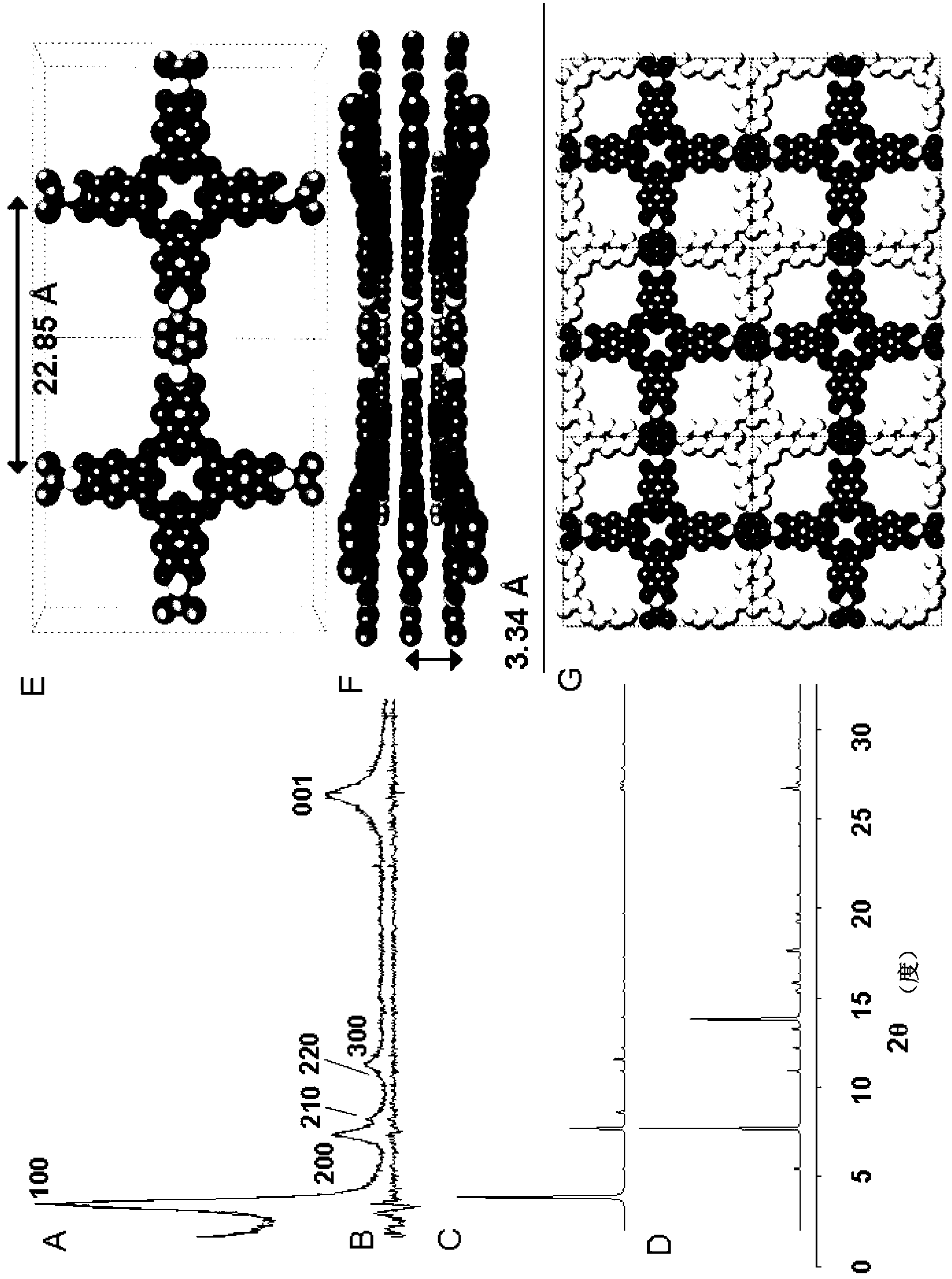
[0148] ZnPc-PDBA COF. Pyrene diboronic acid 1 (17mg, 0.059mmol) and zinc octylhydroxyphthalocyanine 5 (20mg, 0.028mmol) (see Figure 33 ) in a mixture of dioxane and methanol (2:1, 3 mL) and sonicated for 10 min. The dark green suspension was transferred to a 10 mL scored long-neck glass ampoule, snap-frozen in a liquid nitrogen bath, and flame-sealed. The ampoule was placed in a gravity convection oven at 120°C for 96 hours, and the resulting free-flowing dark green powder was collected by filtration through a Hershey funnel, washed with 1 mL of anhydrous toluene, and air-dried. After brief vacuum drying, PXRD and IR characterizations were performed immediately. ZnPc-PDBA COF 10 mg (52%) was isolated. IR (powder, ATR) 3233, 1607, 1459, 1369, 1337, 1271, 1231, 1106, 1078, 1023, 902, 870, 824, 742, 714cm -1 . PXRD [2θ (relative intensity)] 3.22 (100), 6.50 (24), 9...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| size | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com