Preparation method and application for hydrophilic organic polymer liquid phase monolithic chromatographic column
An integral chromatographic column and polymer technology, which is applied in the field of chromatographic research of organic polymer materials, can solve the problems of limited application optimization range, limited polarity of chromatographic column, narrow range of solvent selection, etc., and achieves low cost, less environmental pollution, and preparation The effect of simple process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
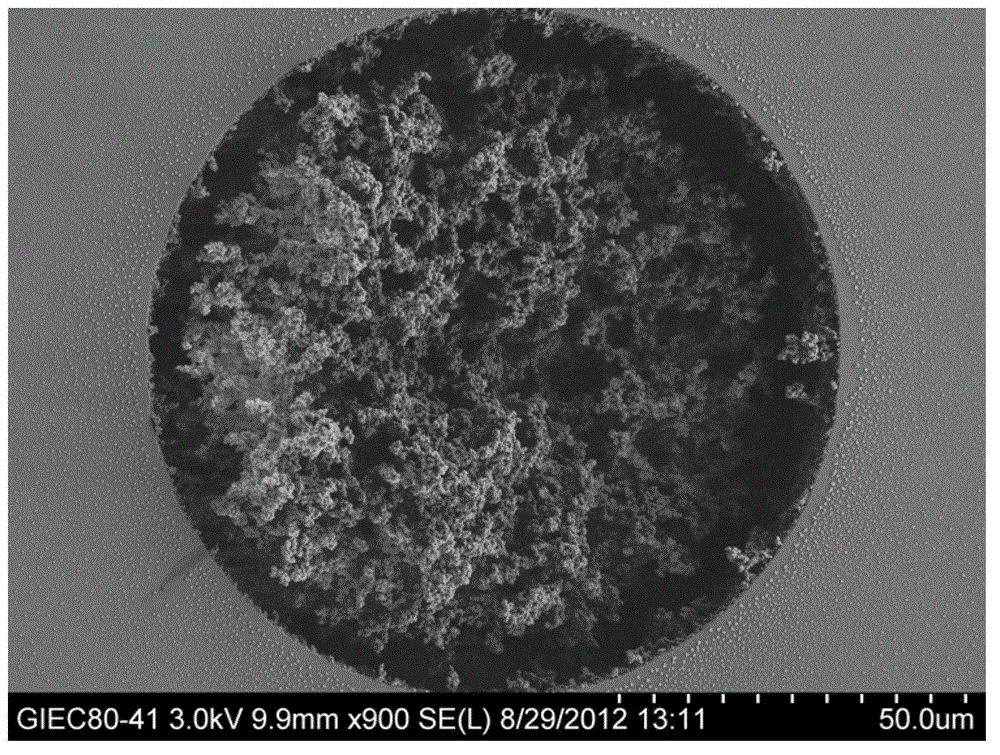
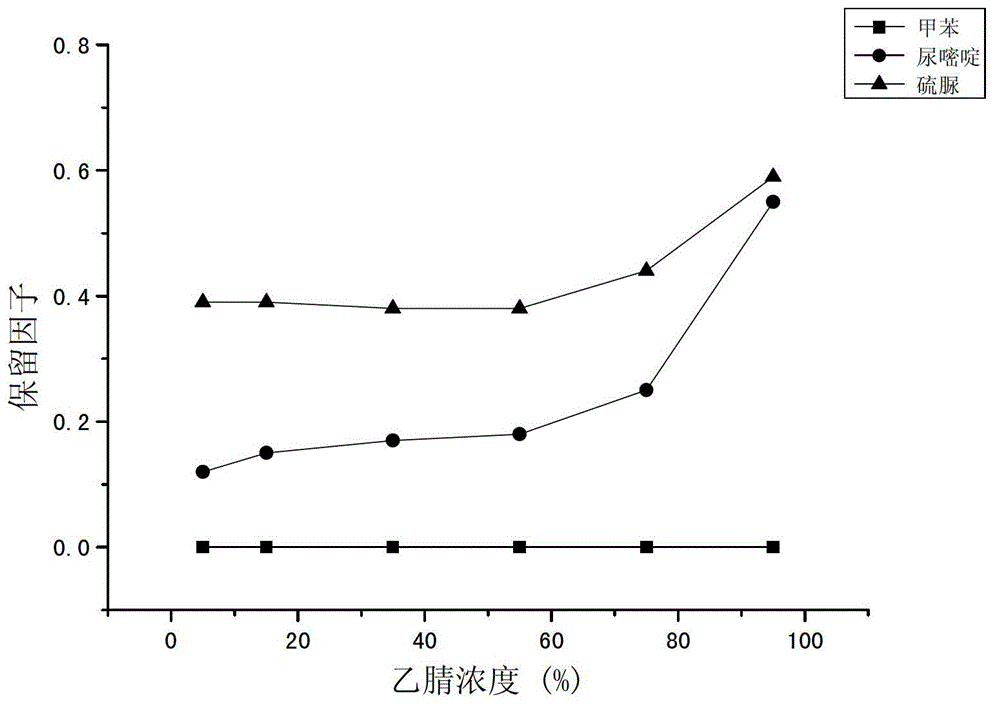
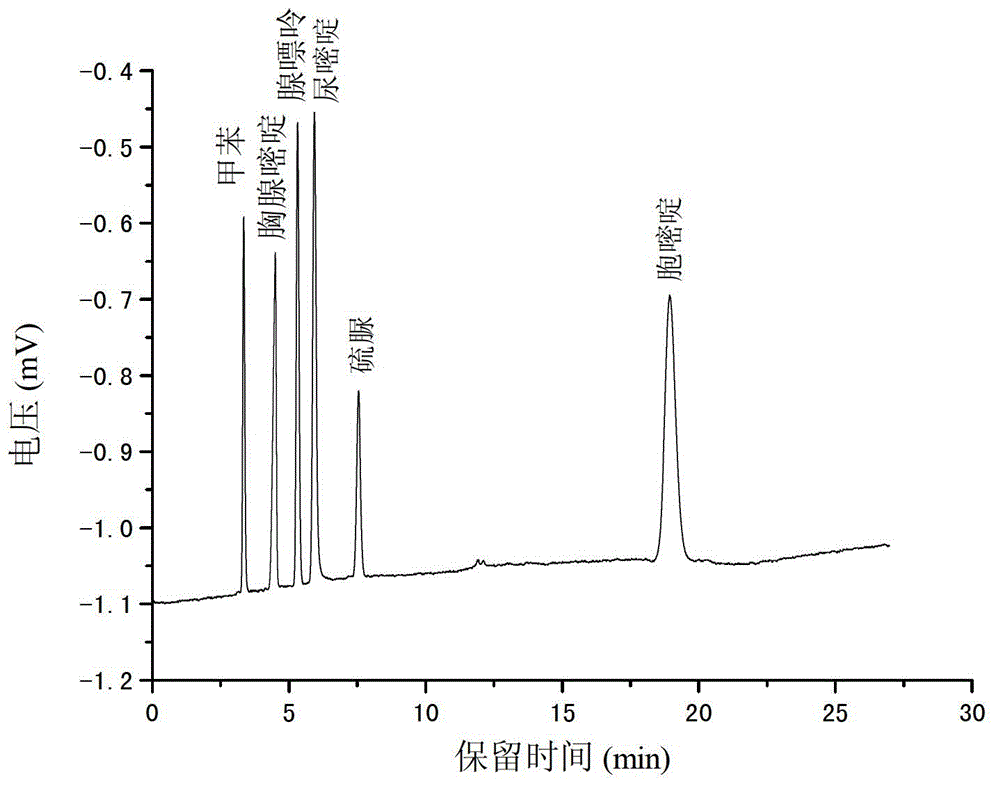
[0026] The monomer 3-[N,N-dimethyl-[2-(2-alkenoyloxy)ethylbutylammonium]propane-1-sulfonic acid inner salt 0.0600g, the crosslinking agent N,N-methylene Base bisacrylamide 0.0106g, porogen (including methanol 0.1128g and water 0.0519g) and initiator azobisisobutyronitrile 0.0010g, prepared into a reaction mixture, ultrasonically degassed and poured into a pretreated quartz capillary Then seal both ends of the quartz capillary and put it in a 60°C water bath to react for 12 hours; after the reaction is completed, connect the quartz capillary to a high-pressure pump, wash off unreacted substances with methanol, and obtain SPDA-co-MBA hydrophilic organic polymer Liquid phase monolithic chromatographic column, the scanning electron microscopy results of the organic polymer in the column are as follows: figure 1 shown.
Embodiment 2
[0028] The monomer 3-[N,N-dimethyl-[2-(2-alkenoyloxy)ethylbutylammonium]propane-1-sulfonic acid inner salt 0.0600g, the crosslinking agent N,N-methylene Base bisacrylamide 0.0257g, porogen (methanol 0.1370g and water 0.0630g) and initiator azobisisobutyronitrile 0.0010g, prepared into a reaction mixture, ultrasonically degassed and poured into a pretreated quartz capillary , and then seal both ends of the quartz capillary, and put it in a 60°C water bath to react for 12 hours. After the reaction is completed, connect the quartz capillary to a high-pressure pump, rinse off unreacted substances with methanol, and obtain SPDA-co-MBA hydrophilic organic polymer Liquid chromatography monolithic columns.
Embodiment 3
[0030] The monomer 3-[N,N-dimethyl-[2-(2-alkenoyloxy)ethylbutylammonium]propane-1-sulfonic acid inner salt 0.0600g, the crosslinking agent N,N-methylene Base bisacrylamide 0.0132g, porogen (methanol 0.1170g and water 0.0538g) and initiator azobisisobutyronitrile 0.0010g, prepared into a reaction mixture, ultrasonic degassed and poured into the pretreated quartz capillary , and then seal both ends of the quartz capillary, and put it in a 60°C water bath to react for 12 hours. After the reaction, connect the quartz capillary to a high-pressure pump, and rinse off unreacted substances with acetone to obtain SPDA-co-MBA hydrophilic organic polymer Liquid chromatography monolithic columns.
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com