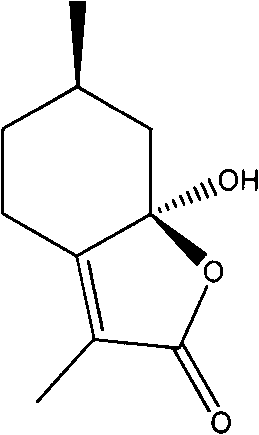
Nepetalactone-o-bromobenzoic acid ester as well as preparation process and use of nepetalactone-o-bromobenzoic acid ester
A technology of benzoate and nepetalactone is applied in the directions of medical preparations containing active ingredients, organic active ingredients, organic chemistry, etc., to achieve the effects of simple and cheap preparation method, simple structure and strong physiological activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] Example 1: Weighing nepetalactone (according to the method disclosed in Chinese patent CN201010217622.1, the commercially available nepeta volatile oil obtained by extracting the flower spikes of the Lamiaceae plant Schizonepeta tenuifolia Briq. by steam distillation is prepared by oxidation And get, purity more than 98%) 18.2mg (0.10mmol), o-bromobenzoic acid 50.2mg (0.25mmol, domestic AR grade), DMAP 12.3mg (0.10mmol, domestic AR grade), it is placed in 50mL round bottom flask Add 4mL of dichloromethane (domestic AR grade), stir to dissolve completely, add DCC 51.6mg (0.25mmol, domestic AR grade), react at room temperature (20-25°C), and check the end point of the reaction by TLC. After the reaction, methanol (domestic AR grade) was added dropwise to clarify the solution, and about 500 mg of silica gel (produced by Qingdao Ocean Chemical Factory, used for column chromatography) was added in three times, and the solvent was distilled under reduced pressure to dryness. ...
Embodiment 2
[0021] Structural analysis of the nepetalactone o-bromobenzoate prepared in Example 1:
[0022] HR-ESI-MS: m / z 366.0415 [M+H] + (calculated value C 17 h 17 BrO 4 is: 365.2185); [α] 20.3 D , +82.5° (c 1.0, CH 3 OH).
[0023] IR (KBr pellet, cm -1 ): 3028 (ν Φ-H ); 2931, 2848 (saturated cyclic hydrocarbon ν CH ); 1754 (lactone carbonyl v C=O ); 1697 (carbonyl v C=O ); 1627, 1572, 1503, 1447 (conjugated benzene ring ν C=C ); 864,792,689 (phenyl ring meta-substituted γ Φ-H ).
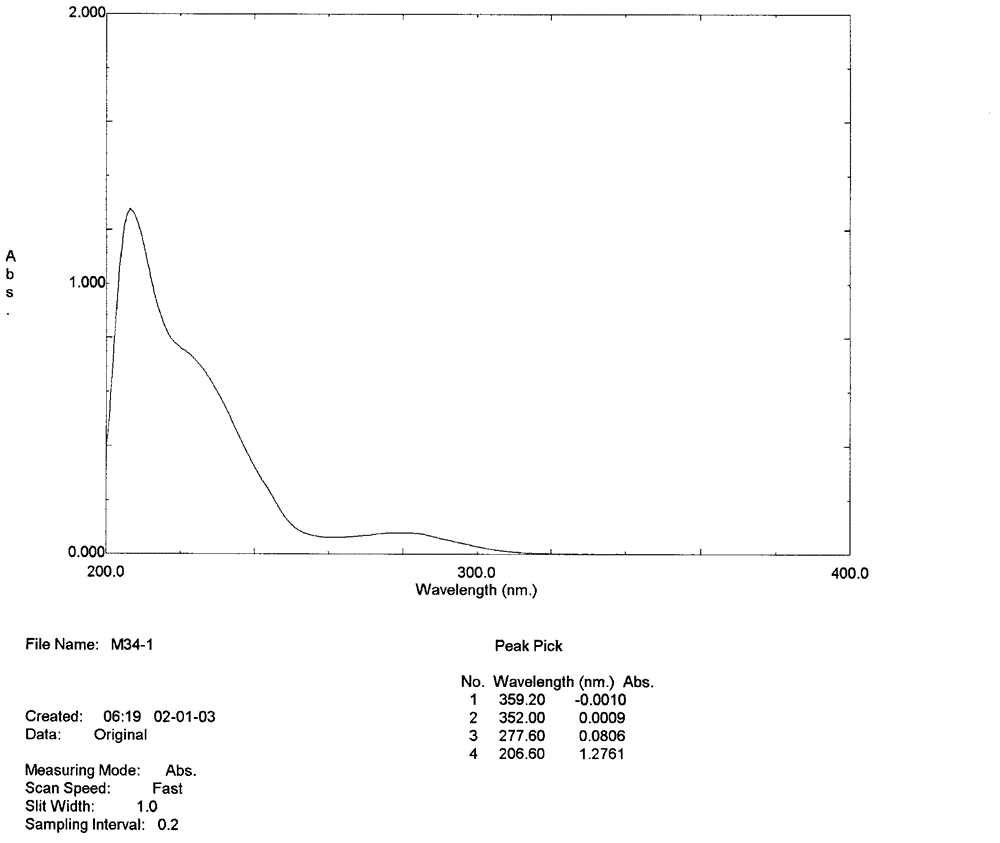
[0024] UV (CH 3 OH)λ max : 277.6nm, suggesting that the compound has conjugated absorption characteristics. See attached figure 1
[0025] h 1 -NMR (300MHz, CDCl 3 , ppm): δ1.01 (d, 3H, J=6.6Hz, 6'-CH 3 ), 1.11(dd, 1H, J=4.2, 13.2Hz, 5a-H), 1.26(m, 1H, 4a-H), 1.90(s, 3H, 3'-H), 1.99-2.10(m, 2H , 5b-H, 6-H), 2.37(m, 1H, 4b-H), 2.79-2.86(m, 2H, 7-H), 7.32-7.39(m, 2H, Ar-H), 7.65(dd , 1H, J=0.9, 4.5Hz, Ar-H), 7.75 (dd, 1H, J=1.2, 4.5Hz, Ar-H).
[0026] 13 C NMR (75MHz, CDCl 3 , ppm): ...
Embodiment 3
[0028]Example 3: Weighing nepetalactone (according to the method disclosed in Chinese patent CN201010217622.1, the commercially available nepeta volatile oil obtained by extracting the flower spikes of the Lamiaceae plant Schizonepeta tenuifolia Briq. by steam distillation is prepared by oxidation And get, purity more than 98%) 18.2mg (0.10mmol), o-bromobenzoic acid 50.2mg (0.25mmol, domestic AR grade), DMAP 12.3mg (0.10mmol, domestic AR grade), it is placed in 50mL round bottom flask Add 4mL of dichloromethane (domestic AR grade), stir to dissolve completely, add EDCI 47.9mg (0.25mmol, domestic AR grade), react at room temperature (20-25°C), and check the end point of the reaction by TLC. After the reaction, methanol (domestic AR grade) was added dropwise to clarify the solution, and about 500 mg of silica gel (produced by Qingdao Ocean Chemical Factory, used for column chromatography) was added in three times, and the solvent was distilled under reduced pressure to dryness. ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com