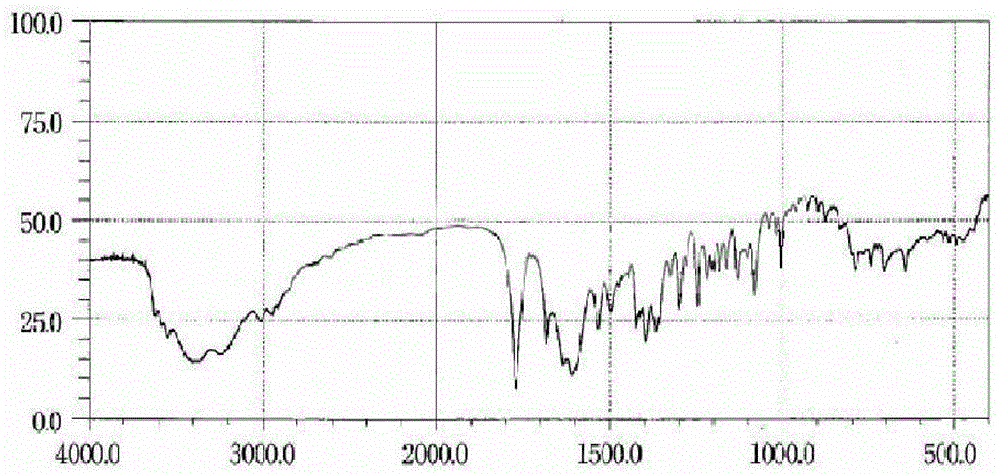
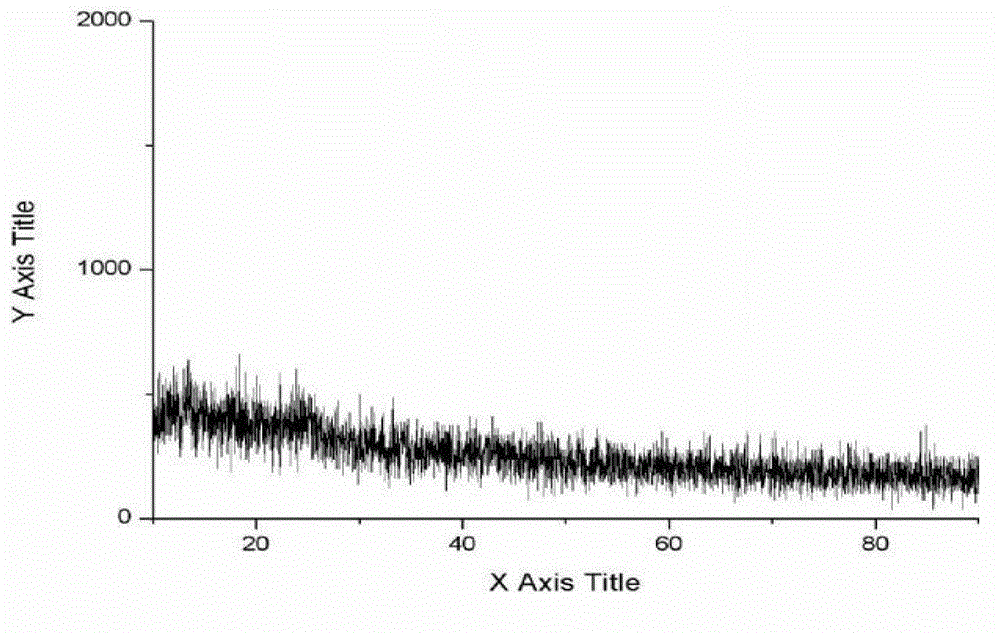
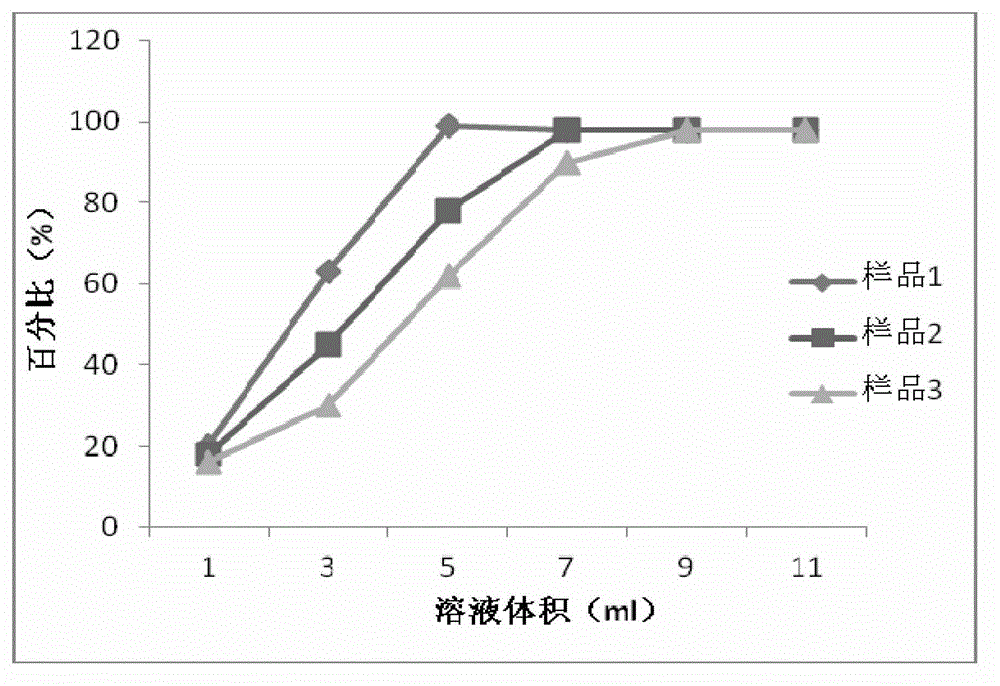
New crystal form composition of cefminox sodium and preparation method thereof
A technology of cefminox sodium and composition, which is applied in the field of medicine and medicine preparation, can solve the problems of slow dissolution rate and poor stability, and achieves the effects of large entropy value, reasonable specification design and good drug compliance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] 1. Prescription:
[0043] Cefminox Acid (95%) 1140g
[0044] Sodium isooctanoate for injection (98%) 332g
[0045] Water for injection 3249ml
[0046] 2. Production process:
[0047]Dissolve 332g of sodium isooctanoate in 2000ml of water and 1140g of cefminox acid in 1249ml of water to form sodium isooctanoate solution and cefminox acid solution according to aseptic operation requirements, slowly add sodium isooctanoate solution into cefminox acid solution (The water temperature of the above two solutions is controlled at 5-8°C), and continuously stirred for 30 minutes. After the reaction is complete, add 0.1% activated carbon and stir to adjust the pH to 4.5-6.0. Freeze-dry the large plate (the liquid level height is 3-4cm), and immediately send it to the vacuum freeze-drying box to freeze-dry. Keep warm for 2 hours, keep the vacuum in the box at 10-20Pa, then start to heat up, and the product temperature will rise to 0°C within 20 hours, then increase the temperat...
Embodiment 2
[0052] 1. Prescription
[0053] Cefminox Acid (95%) 1140g
[0054] Sodium isooctanoate for injection (98%) 332g
[0055] Water for injection 3249ml
[0056] 2. Production process:
[0057] Dissolve 332g of sodium isooctanoate in 2000ml of water and 1140g of cefminox acid in 1249ml of water to form sodium isooctanoate solution and cefminox acid solution according to aseptic operation requirements, slowly add sodium isooctanoate solution into cefminox acid solution (The water temperature of the above two solutions is controlled at 5-8°C), and continuously stirred for 30 minutes. After the reaction is complete, add 0.1% activated carbon and stir to adjust the pH to 4.5-6.0. Freeze-dry the large plate (the liquid level is 3-4cm), and immediately send it to the vacuum freeze-drying box for freeze-drying. First, the temperature of the plate layer is lowered to below -45°C, and then the product is sent into the product. The temperature of the product reaches -50°C within 2 hours. ...
Embodiment 3
[0060] Preparation of cefminox sodium new crystal form composition composition powder injection: take by weighing 500 g of cefminox sodium new crystal form composition prepared in Example 1, 15 g of mannitol, sterilize by ultraviolet light, mix, and then aseptic raw materials Put it into the filling machine, adjust the filling amount according to the specification of 0.5g / bottle (calculated as cefminol (C16H21N7O7S3)), fill it into bottles after passing the inspection, buckle the butyl rubber stopper, roll the cap, and pack the finished product into the warehouse and send it check.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com