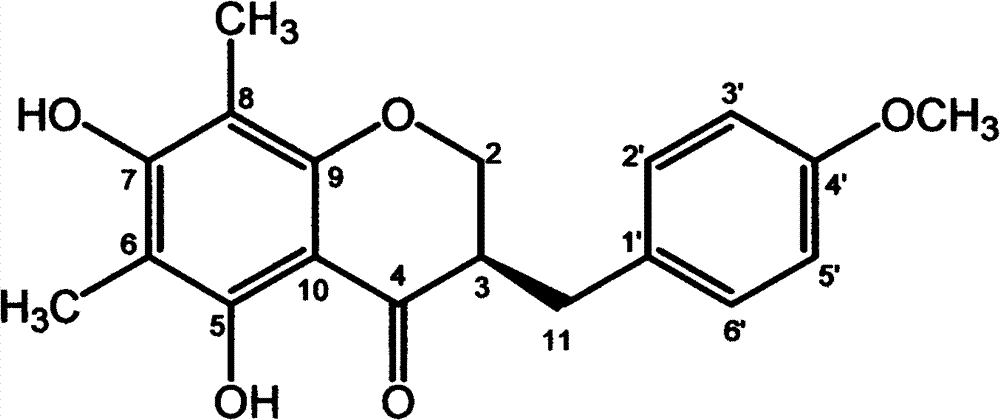
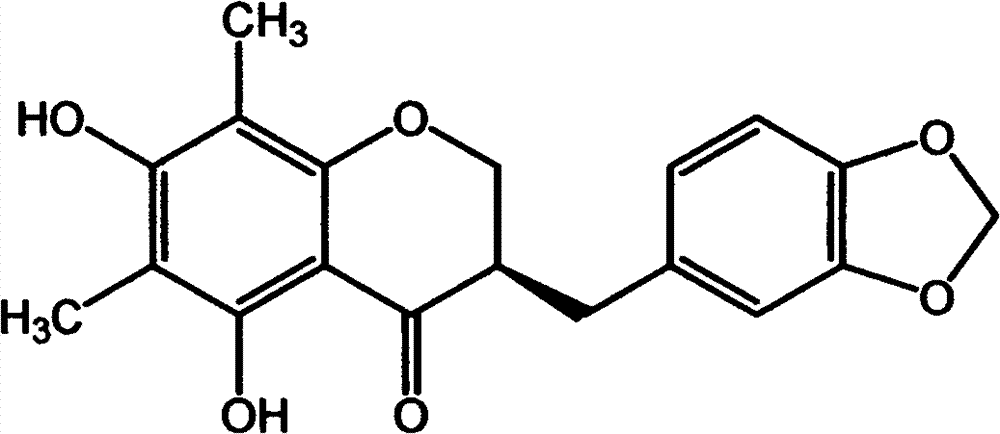
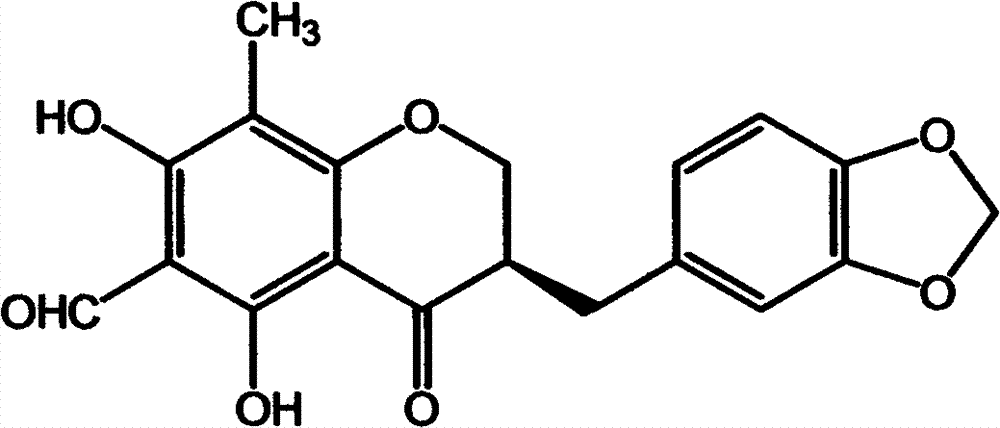
Isoflavones composition and medicine made of same
A technology of isoflavones and methyl isoflavones, which is applied in the field of medicine and can solve the problems of large toxic and side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0057] Anti-leukemia Drug Efficacy Test in Mice
[0058] 1. Test method
[0059] The mouse lymphocytic leukemia cell line L1210 was subcultured in DMEM (GIBCO) medium containing 15% calf serum, and 20%-30% volume of fresh medium was added every 2-4 days. Culture conditions: 5% CO2, saturated humidity, 37°C.
[0060] The animals used were DBA / 2 inbred mice, 8-12 weeks old, half male and half male. Bought from Shanghai Slack Experimental Animal Co., Ltd. [SCXK (Shanghai) 2003-0003], take cultured cells in logarithmic growth phase, centrifuge at 1500r / min for 5min, discard supernatant, resuspend cells in serum-free DMEM, centrifuge at 1500r / min for 5min , Cell counting, each mouse was intraperitoneally injected with 0.1ml of cell suspension (containing 1×106 cells). DBA / 2 mice were randomly divided into 5 groups after inoculation: blank control group, positive drug doxorubicin (ADM) group (2mg / kg) and total flavonoids group (120mg / kg), two component groups ( 80mg / kg) and sing...
Embodiment 2
[0071] Drug Efficacy Test of Anti-transplanted Lung Cancer in Mice
[0072] 1. Test method
[0073] Mouse Lewis lung cancer cells cryopreserved in liquid nitrogen were resuscitated, and subcutaneously inoculated 5×105 / cell in the right axilla of 4 C57BL / 6 mice to prepare passage tumor-bearing mice. The mice were sacrificed after 3 passages, and the tumor mass of the tumor-bearing mice was stripped under aseptic conditions to prepare a 5×106 / mL tumor cell suspension, and then 0.2 mL of tumor cells were subcutaneously inoculated into the right axilla of mice in each group suspension. Stratified random grouping according to body weight, respectively blank control group, positive drug cisplatin (DDP) group (1mg / kg) and total flavonoids group (120mg / kg), two component groups (80mg / kg) and single component group ( 50mg / kg). 10 animals per group.
[0074] 7 days after inoculation, when the tumor grows to a volume of 100-200mm3, the drug is administered. The drug of the present inve...
Embodiment 3
[0085] Anti-transplanted Prostate Cancer Drug Efficacy Test
[0086] 1. Test method
[0087] Passaging in vivo: Human prostate cancer PC-3M cell line (purchased from Sino-US joint venture Bohuisi Biomedical Technology Co., Ltd.) cultured in vitro in logarithmic growth phase was digested with trypsin to prepare cell suspension, and the concentration was adjusted to 1× 107 / ml, inoculated subcutaneously in the right axilla of mice, 0.2mL / only. Three generations were passed continuously in vivo, and the tumor-bearing mice of the third generation were used as the tumor source for the experiment.
[0088] Inoculation: 2 weeks after inoculation, take well-grown human prostate cancer PC-3M tumor blocks in nude mice, add physiological saline, grind with a homogenizer to prepare a cell suspension, count and adjust the number of cells to 1×107 / ml, Each nude mouse was subcutaneously inoculated with 0.2ml in the right armpit, and stratified and randomly divided into groups according to b...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com