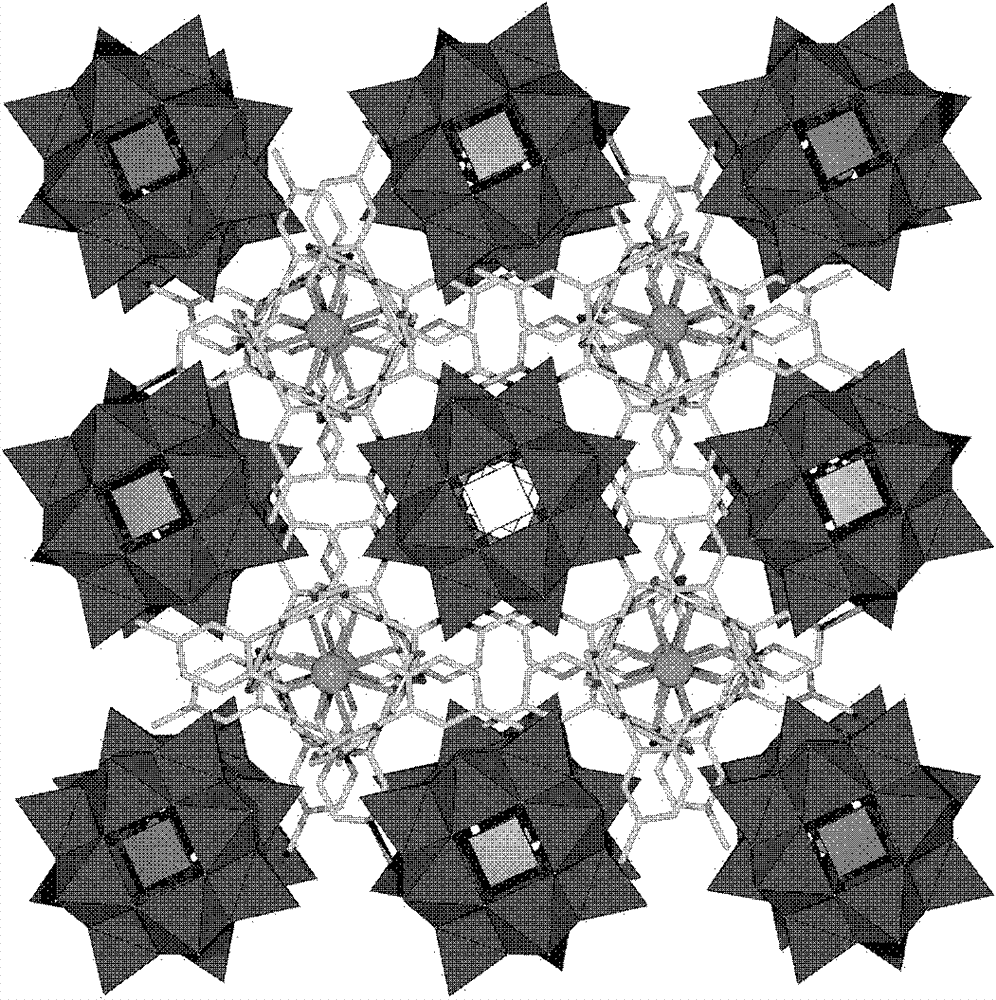
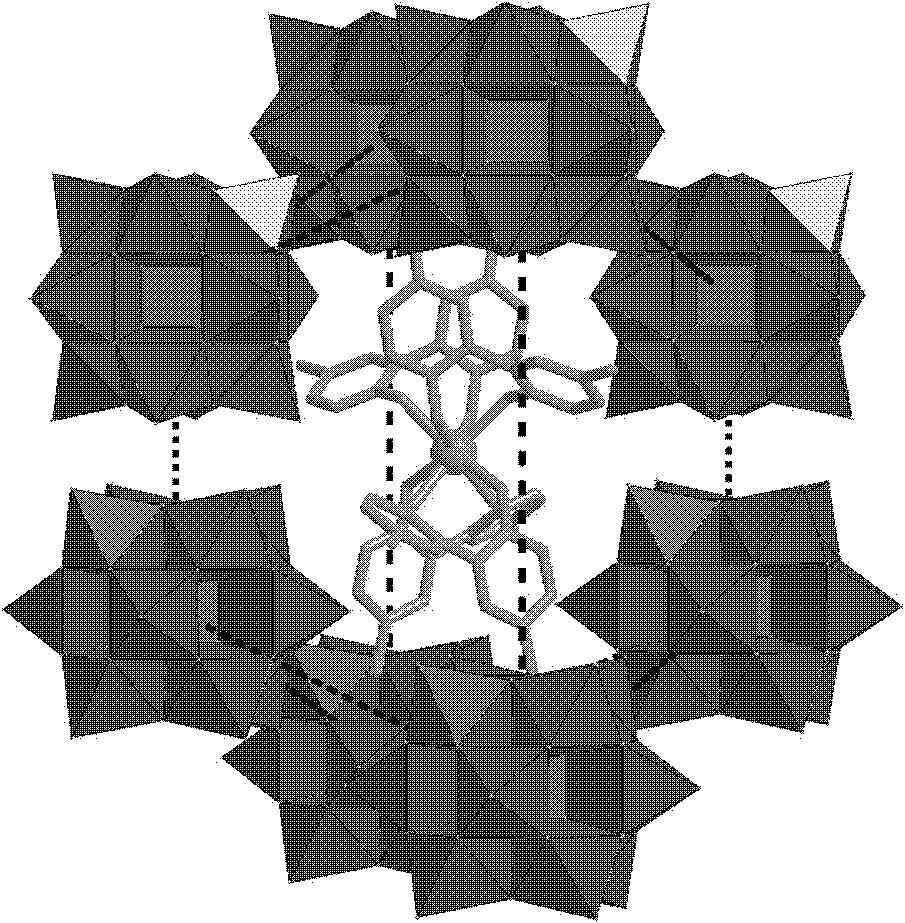
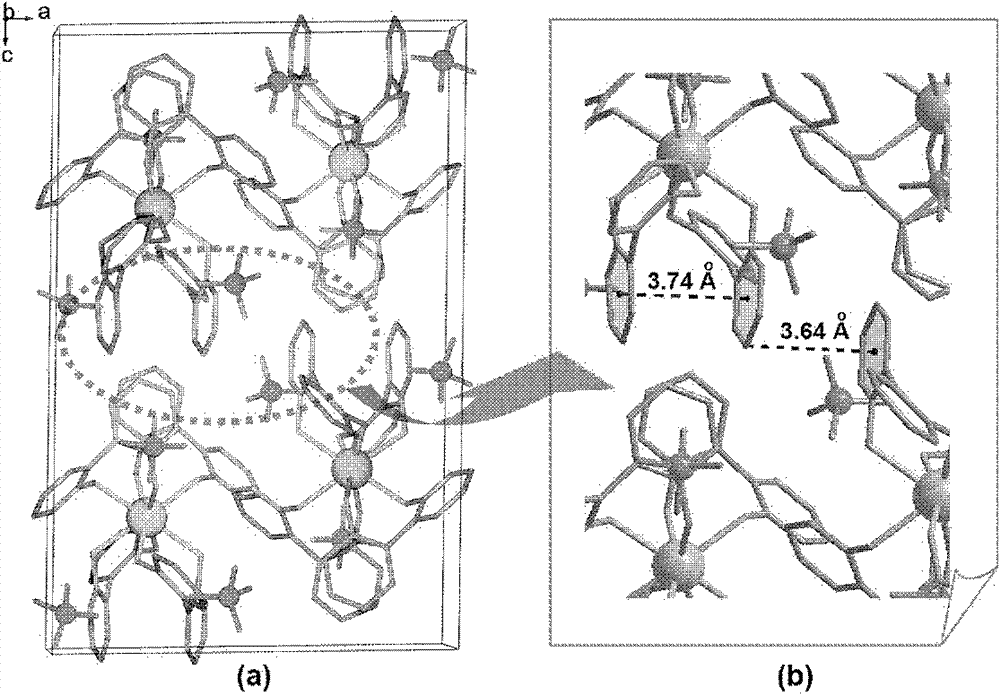
Preparation method of polyacid rare earth ion complex with slow magnetic relaxation behavior
A technology of rare earth ions and magnetic relaxation, which is applied in organic chemistry and other fields, can solve the problems of slow magnetic relaxation, and achieve the effects of good repeatability, high yield and stable preparation method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] (1) NdPMo 12 o 40 ·nH 2 The preparation of O: the α-H 3 PMo 12 o 40 14H 2 O (410mg, 0.2mmol) and NdCl 3 ·6H 2 O (80mg, 0.2mmol) was dissolved in a small beaker containing 20mL of water, stirred, heated, and the solvent was evaporated until light yellow crystals were precipitated, then cooled. The yield was 65% (calculated as Mo).
[0024] (2) Preparation of 4,4'-dimethyl-2,2'-bipyridine-N-N'-dioxide (bpyno for short): 4,4-dimethyl-2,2-bipyridine (5.4 g, 25mmol), glacial acetic acid (10mL) and 35% aqueous hydrogen peroxide (10mL) were placed in a three-necked flask equipped with a reflux condenser, and heated in a water bath at 70-80°C for 3 hours. Thereafter, 35% aqueous hydrogen peroxide (7.5 mL) was added to the reaction system, and heating was continued for 9 hours. After cooling, acetone (75 mL) was added to obtain a pale yellow precipitate, which was filtered with suction and washed twice with acetone. The product was dried in vacuum and the yield was 58...
Embodiment 2
[0027] (1) SmPMo 12 o 40 ·nH 2 The preparation of O: the α-H 3 PMo 12 o 40 14H 2 O (410mg, 0.2mmol) and SmCl 3 ·6H 2 O (80mg, 0.2mmol) was dissolved in a small beaker containing 20mL of water, stirred, heated, and the solvent was evaporated until light yellow crystals were precipitated, then cooled. The yield was 65% (calculated as Mo).
[0028] (2) Preparation of 4,4'-dimethyl-2,2'-bipyridine-N-N'-dioxide (bpyno for short): 4,4-dimethyl-2,2-bipyridine (5.4 g, 25mmol), glacial acetic acid (10mL) and 35% aqueous hydrogen peroxide (10mL) were placed in a three-necked flask equipped with a reflux condenser, and heated in a water bath at 70-80°C for 3 hours. Thereafter, 35% aqueous hydrogen peroxide (7.5 mL) was added to the reaction system, and heating was continued for 9 hours. After cooling, acetone (75 mL) was added to obtain a pale yellow precipitate, which was filtered with suction and washed twice with acetone. The product was dried in vacuum and the yield was 58...
Embodiment 3
[0031] (1) EuPMo 12 o 40 ·nH 2 The preparation of O: the α-H 3 PMo 12 o 40 14H 2 O (410mg, 0.2mmol) and EuCl 3 ·6H 2 O (80mg, 0.2mmol) was dissolved in a small beaker containing 20mL of water, stirred, heated, and the solvent was evaporated until light yellow crystals were precipitated, then cooled. The yield was 65% (calculated as Mo).
[0032] (2) Preparation of 4,4'-dimethyl-2,2'-bipyridine-N-N'-dioxide (bpyno for short): 4,4-dimethyl-2,2-bipyridine (5.4 g, 25mmol), glacial acetic acid (10mL) and 35% aqueous hydrogen peroxide (10mL) were placed in a three-necked flask equipped with a reflux condenser, and heated in a water bath at 70-80°C for 3 hours. Thereafter, 35% aqueous hydrogen peroxide (7.5 mL) was added to the reaction system, and heating was continued for 9 hours. After cooling, acetone (75 mL) was added to obtain a pale yellow precipitate, which was filtered with suction and washed twice with acetone. The product was dried in vacuum and the yield was 58...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com