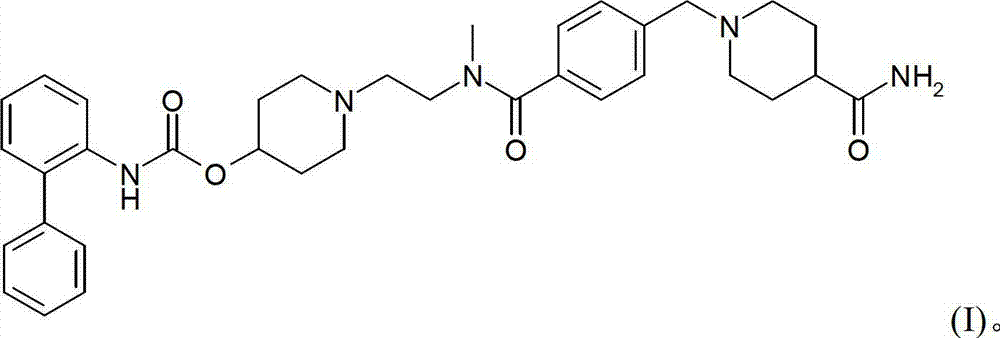
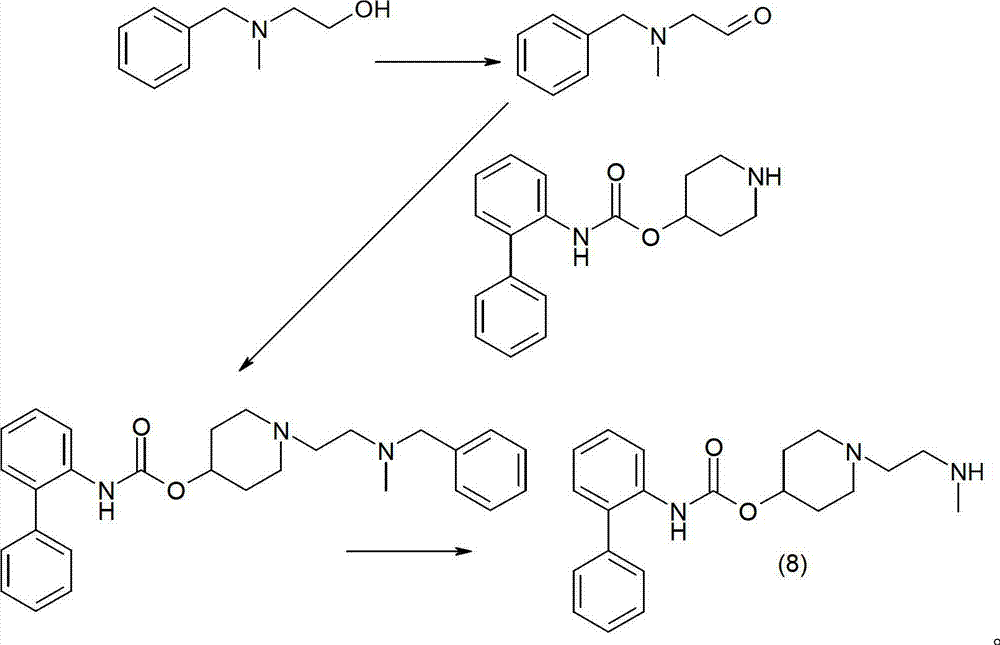
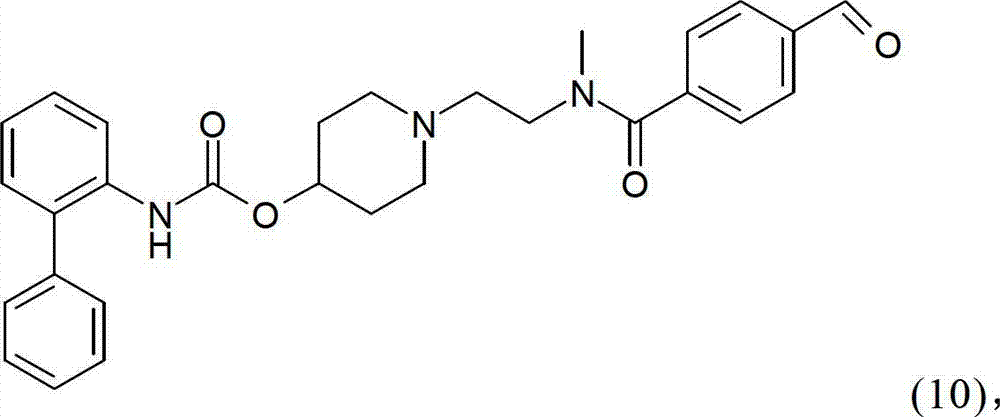
Process for preparing a biphenyl-2-ylcarbamic acid
A technology of methyltetrahydrofuran and compound, applied in the preparation of the crystalline free base of this ester, the preparation of intermediates that can be used for synthesizing the ester and the crystalline free base, and can solve the problem of low yield, difficult to scale up, and aldehyde intermediates. instability, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example
[0095] The following Preparations and Examples are provided to illustrate specific examples of the invention. However, unless expressly stated otherwise, the specific examples are not intended to limit the scope of the invention in any way. Unless otherwise stated, the following abbreviations have the following meanings, and any other abbreviations used herein and not defined have their standard meanings:
[0096] AcOH acetic acid
[0097] EtOAc ethyl acetate
[0098] EtOH: ethanol
[0100] iPrOAc isopropyl acetate
[0101] MeCN acetonitrile
[0102] MeOH Methanol
[0103] MTBE methyl tert-butyl ether
[0104] MeTHF 2-Methyltetrahydrofuran
[0105] NaHB(OAc) 3 Sodium triacetoxyborohydride
[0106] Any other abbreviations used herein but not defined have their accepted standard meanings. Reagents, starting materials, and solvents were purchased from commercial suppliers (eg, Sigma-Aldrich, Fluka, and the like) and used without further...
example 1
[0112] Step A: Benzyl (2,2-Dimethoxyethyl)methylcarbamate
[0113]
[0114] mixed K 2 CO 3 (13.8g, 100mmol, 1.76eq.) with H 2 O (46 mL) to form a homogeneous solution. The solution was cooled to 20°C. N-Methylaminoacetaldehyde dimethyl acetal (12.8 mL, 100 mmol, 1.8 eq) and MeTHF (50 mL) were added. The resulting mixture was cooled to 2°C. Benzylchloroformate (8.1 mL, 56.7 mmol, 1.0 eq.) was added via syringe over 10 minutes (addition was exothermic). The mixture was maintained at room temperature until the reaction was complete. The layers were separated and the organic layer was washed with 1N HCl (50 mL) and used directly in the next step.
[0115] Step B: Benzyl Methyl-(2-oxoethyl)carbamate
[0116]
[0117] The mixture from the previous step was combined with 3N HCl solution (70 mL) and the resulting mixture was stirred at 22 °C for 18 hours to yield a clear homogeneous pale yellow solution. Solid NaHCO 3 added to the solution to neutralize the pH. The la...
example 2
[0127] All volumes and molar equivalents are given relative to biphenyl-2-yl-piperidin-4-ylcarbamate.
[0128] Step A: Benzyl (2,2-Dimethoxyethyl)methylcarbamate
[0129] Will K 2 CO 3 (8.4kg, 60mol, 1.8eq.) and H 2 O (49.3 kg, 2.6 vol) was placed in the reaction vessel and stirred. N-Methylaminoacetaldehyde dimethyl acetal (6.5 kg, 54 mol, 1.6 eq) and MeTHF (20.2 kg, 2.9 vol) were added. The resulting mixture was cooled to 5°C. Benzyl chloroformate (6.8 kg, 37.6 mol, 1.1 eq.) was added over a period of about 30 minutes while maintaining the temperature below 10°C. The feed tube was flushed with MeTHF (4.3 kg). The mixture was then maintained at 5°C and stirred for 1 hour. The layers were separated and the organic layer was washed with 1N HCl (14.3 kg, 11.7 mol, 1.4 vol) and used directly in the next step.
[0130] Step B: Benzyl Methyl-(2-oxoethyl)carbamate
[0131] The mixture from the previous step was combined with water (23.4 kg, 2.9 vol) and 30% hydrochloric aci...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com