Method for preparing 1,1,1,3,3,3-hexafluoroacetone through gas-phase catalysis
A technology for the catalytic preparation of hexafluoroacetone, which is applied to the preparation of carbon-based compounds, chemical instruments and methods, and the preparation of organic compounds. Low cost, simple process, environment-friendly effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
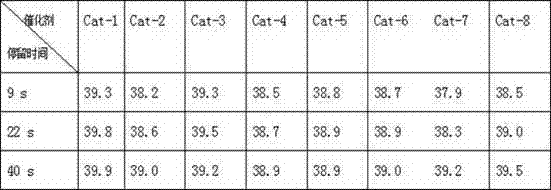
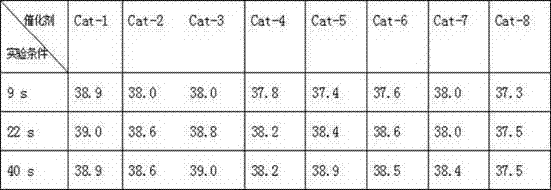
[0020] Mix 500 mL of 36.5% hydrochloric acid and 500 mL of 40% hydrofluoric acid to form an acid solution. Pour 150 g of activated carbon particles with a diameter of 2-4mm into the above acidic solution, stir at room temperature for 24 hours, filter, wash with water to neutrality, dry at 120°C for 10 hours, and dry at 300°C for 5 hours, as a catalyst carrier for later use .
[0021] Add 5 g CrCl 3 And 0.5g LaF 3 Dissolve in 50 mL of water to make a solution, weigh 44.5 g of the catalyst carrier and pour into the solution, and stir for 3 hours. Dry for 5 hours at 120°C and 5 hours at 300°C to obtain 10wt% CrCl 3 / 1wt%LaF 3 / C catalyst, denoted as Cat-1.
Embodiment 2
[0023] Prepare 10wt% Cr in the same way as in Example 1. 2 O 3 / 1wt%LaF 3 / C and marked as Cat-2, the difference is that Cr is used 2 O 3 Instead of CrCl 3 .
Embodiment 3
[0025] Prepare 10wt%CrBr in the same way as in Example 1. 3 / 1wt%LaF 3 / C and marked as Cat-3, the difference is that CrBr is used 3 Instead of CrCl 3 .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com