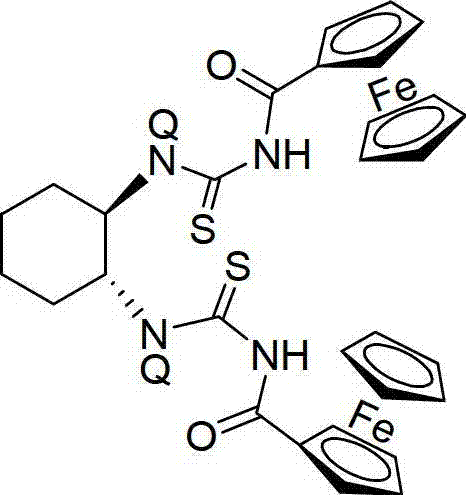
Chiral bisferrocene acylthiourea compound as well as synthesis method and application of compound
A technology of bisferrocenylthiourea and ferrocenylthiourea, applied in the preparation of anticancer drugs, in the field of chiral bisferrocenylthiourea compounds, can solve the problem of bis(acylthiourea) The problem of fewer compounds, etc., achieves the effect of simple synthesis method and easy separation and purification
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] Example 1 Preparation of chiral bisferrocenylthiourea compound of the present invention
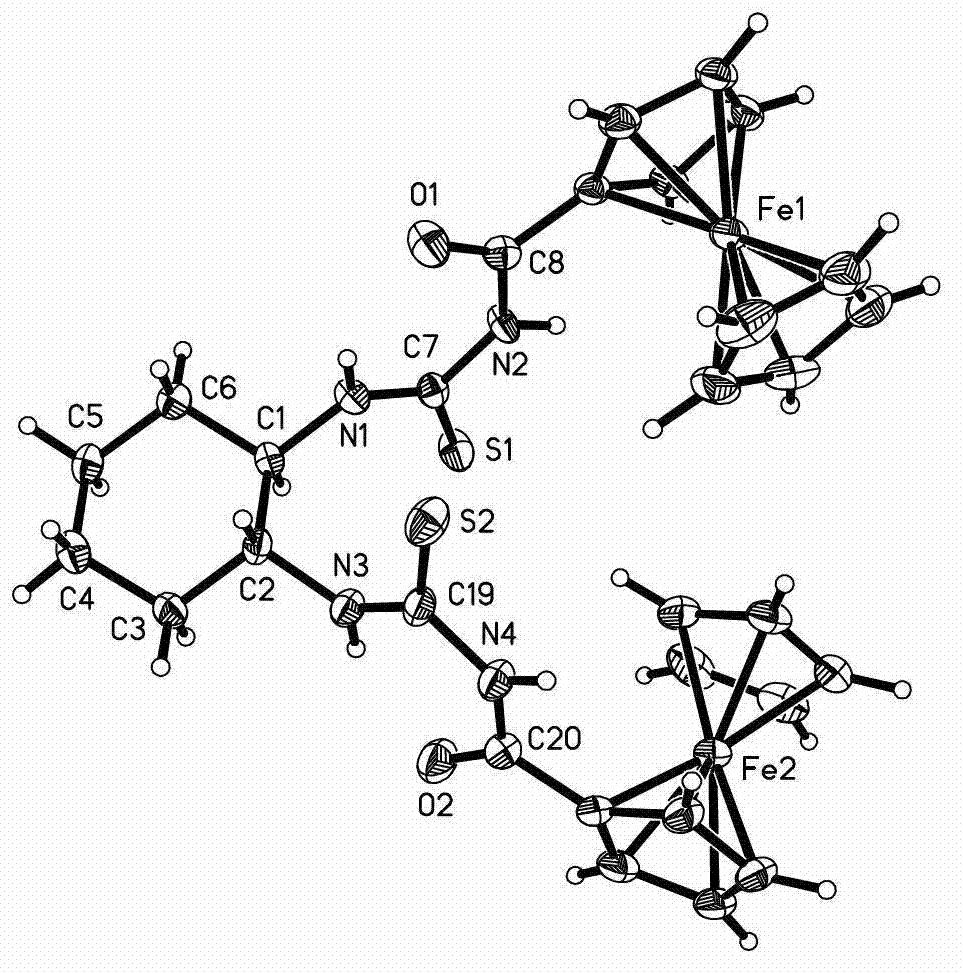
[0021] (1) In a three-neck round bottom flask equipped with a condenser and a stirrer, add 4.08g (17.7mmol) of ferrocenecarboxylic acid and 70ml of anhydrous benzene and stir to obtain a yellow suspension. Add 2.83g (1.8 ml, 20.6 mmol) phosphorus trichloride, and then the reaction mixture was stirred under heating in a water bath at 55-60°C. The ferrocenecarboxylic acid was gradually dissolved, and the solution turned from yellow to dark red, and the reaction lasted for 3 hours. After cooling, the reaction mixture was poured into a distillation flask, the solvent was removed under reduced pressure, and the residue was extracted with 60ml of petroleum ether after cooling. The solvent was removed from the extract under reduced pressure, and cooled to obtain 4.13 g of ferrocenecarbonyl chloride.
[0022] (2) Dissolve 4.13g (16.6mmol) of ferrocenecarbonyl chloride in 30ml of anhydrou...
Embodiment 2
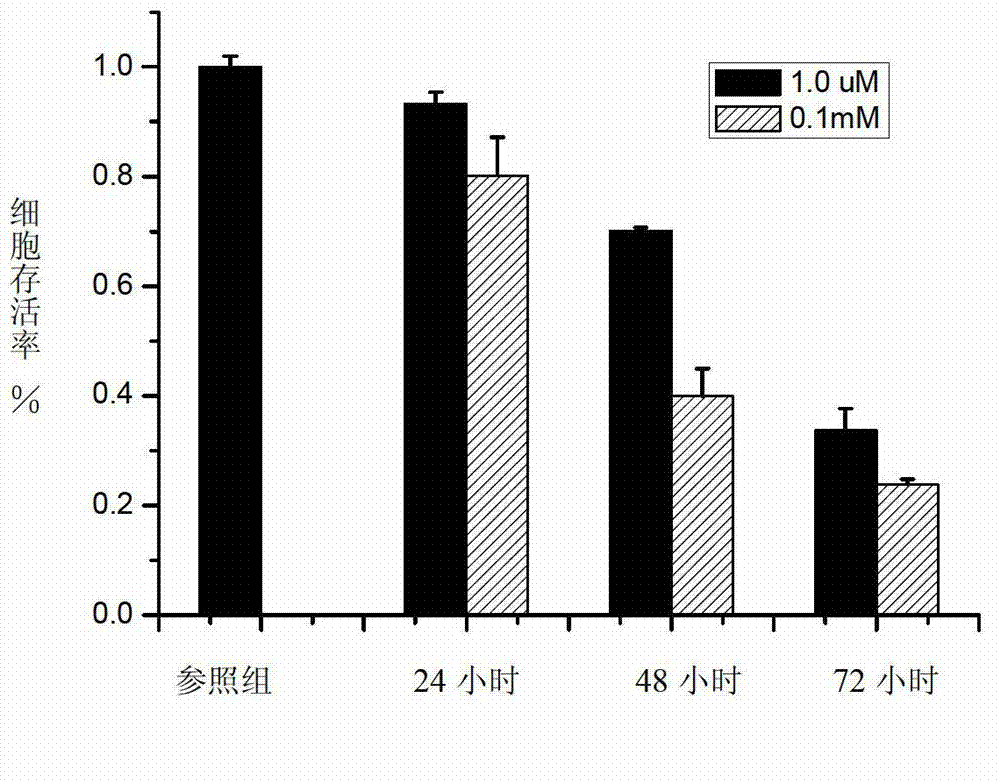
[0028] Example 2 Anticancer activity experiment of chiral bisferrocenylthiourea compound of the present invention
[0029] 1) Cell model: liver cancer cell (HepG2) was used as the model.
[0030] 2) Detection method: MTT colorimetric method to detect anticancer activity test:
[0031] Taking liver cancer cells (HepG2) as a model, the effect of the chiral bisferrocenylthiourea compound (Q=H in the general structural formula) of the present invention on its biological activity was studied. Take the HepG2 cell suspension (7.5×10 3 cells / mL) were inoculated in 96-well cell culture plate, 200 μL per well, placed at 37C, 5% CO 2 Incubate in the incubator for 16 hours. After the cells are completely adhered to the wall, add different concentrations of ligands (1.0 μM and 0.1 mM) and incubate for 24, 48 and 72 hours. Use untreated blank cells as the control, and set 6 wells for each group of experiments. Repeat hole. After the reaction, add 20 μL MTT (5 mg / mL) to each well, contin...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com