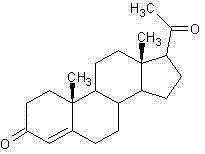
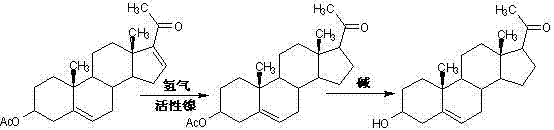
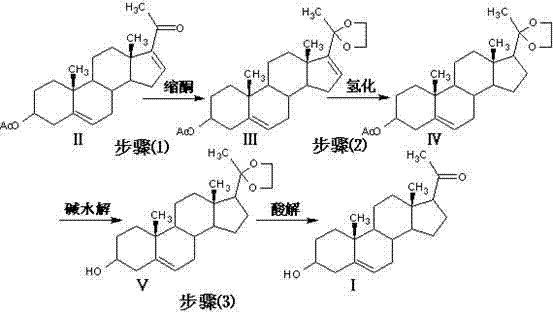
Method for synthesizing progesterone midbody 3beta-hydroxy-5-pregnene-20-ketone
A synthesis method, the technology of pregnene, which is applied in the field of synthesis of pharmaceutical intermediates, can solve the problems of large amount of catalyst active nickel and difficult control, and achieve the effects of reducing refining processes, improving safety, and easy reaction control
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] 1. Preparation of compound 3β-hydroxy-5,16-pregnadiene-3-acetate-20,20-ethylenedioxy (Ⅲ)
[0032] First, add 450kg of dichloromethane, 150kg of triethyl orthoformate, 150kg of ethylene glycol, 50kg of dienolone acetate, and 2.5kg of pyridine hydrochloride into the reactor, stir well and keep warm at 20°C React for 5 hours; after the reaction is completed, lower the temperature to below 5°C, start adding triethylamine dropwise, control the temperature at 5°C to 10°C, and stir for 30 minutes after adding; Methanol entrainment, after evaporating methanol to dryness, add 50kg of methanol and reflux for 30 minutes; under reflux, add 150kg of pure water dropwise, and then reflux for 1 hour after adding; cool down to below 5°C, continue to stir for 30 minutes, and let it stand for more than 2 hours , centrifuged to dry, washed with water and dried to obtain 55.7kg of compound (Ⅲ).
[0033] 2. Preparation of compound 3β-hydroxy-5-pregnene-3-acetate-20,20-ethylenedioxy (IV)
...
Embodiment 2
[0038]1. Preparation of compound 3β-hydroxy-5,16-pregnadiene-3-acetate-20,20-ethylenedioxy (Ⅲ)
[0039] First, 550kg of chloroform, 150kg of triethyl orthoformate, 180kg of ethylene glycol, 50kg of dienolone acetate, and 1.8kg of p-toluenesulfonic acid were added to the reactor, stirred evenly and placed in the reactor at 25 ℃ for 4 hours to react; after the reaction is completed, lower the temperature to below 5 ℃, start adding triethylamine dropwise, control the temperature at 5 ℃ ~ 10 ℃, and stir for 30 minutes after the addition; evaporate all solvents under reduced pressure, and then use Entrained with 20kg of methanol, evaporated to dryness, added 50kg of methanol and refluxed for 30 minutes; under reflux, added dropwise 150kg of pure water, and refluxed for 1 hour after addition; cooled to below 5°C, continued to stir for 30 minutes, and stood for 2 After more than 1 hour, it was centrifuged to dry, washed with water and dried to obtain 55.5kg of compound (Ⅲ).
[0040]...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com