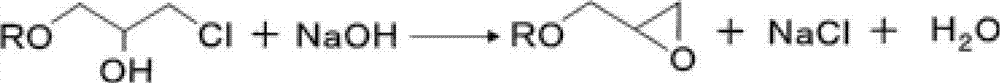Method for producing alkylglycidyl ether
A technology of glycidyl ether and a manufacturing method, which is applied in chemical instruments and methods, electrolysis process, organic chemistry and other directions, can solve the problems of by-produced metal salts, a large amount of water resources, etc., so as to reduce the generation of waste water and effectively utilize water resources. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0116] To 60.0 g of the composition (A1), 12.0 g of sodium chloride and 528.0 g of ion-exchanged water were added to obtain a chlorohydrin ether-containing composition (A) (containing 42.6 g of chlorohydrin ether).
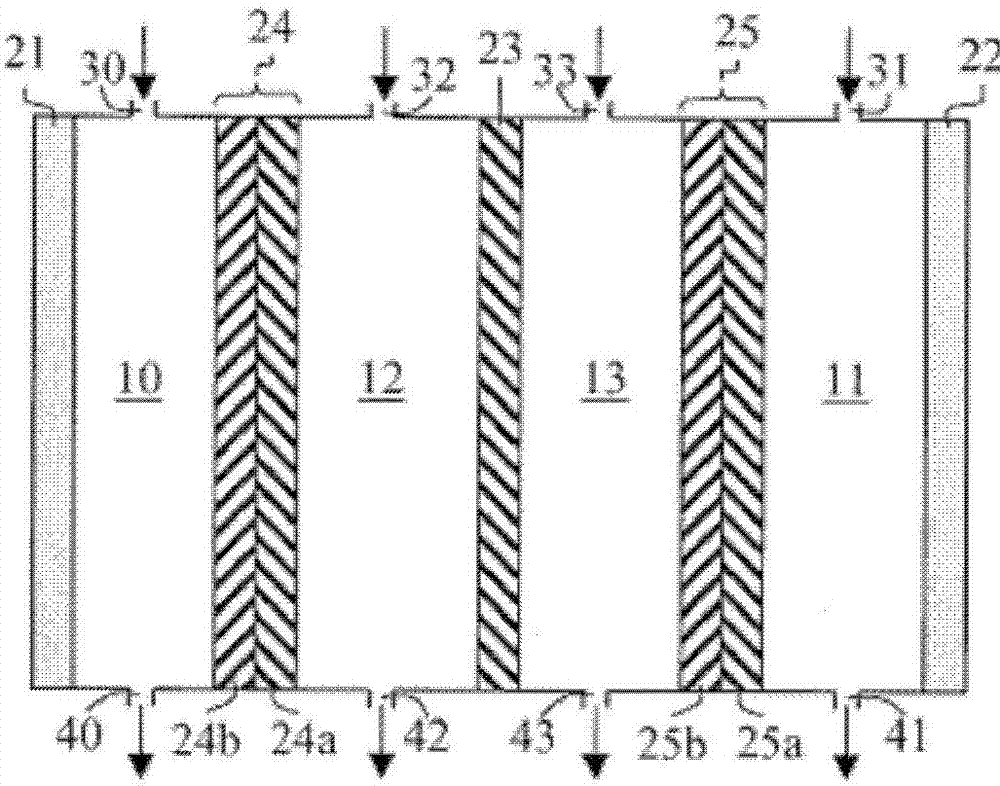
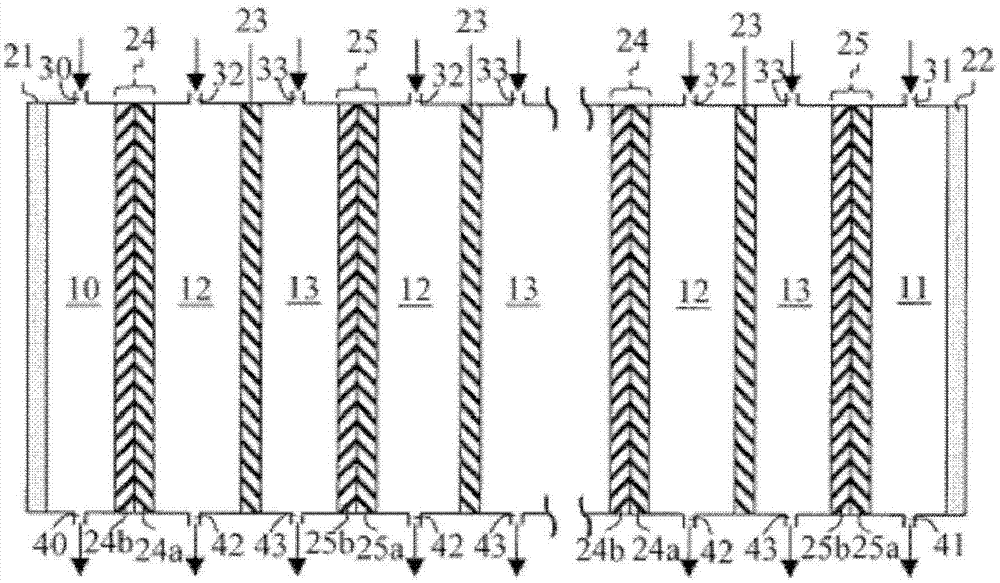
[0117] Step 1: 10 sheets of anion exchange membranes (AHA manufactured by ASTOM Corporation, effective area 55 cm) 2 ), 11 bipolar membranes (BP-1E manufactured by ASTOM Corporation, effective area 55cm 2 )according to figure 2 Composition (A) containing chlorohydrin ethers in zone 1 of the alternately arranged electrodialysis apparatus (Model EX3B manufactured by ASTOM Corporation), or in zone 2, will consist of 10.0 g of sodium chloride and 490.0 g of ion exchanged The electrolyte aqueous solution composed of water was circulated with a pump under stirring, and the voltage between the cathode and the anode was kept constant at 30V, while a direct current was applied, and the electrodialysis reaction was carried out for 2 hours. The cumulative current flowing ...
Embodiment 2
[0121] In addition to adding 12.0 g of sodium chloride and 438.0 g of ion-exchanged water to 150.0 g of composition (A1) to obtain a chlorohydrin ether-containing composition (A) (containing 106.5 g of chlorohydrin ether), and in step 1, The composition (A) containing the chlorohydrin ether was circulated in the area 1 of the electrodialysis apparatus with a pump under stirring, and the electrodialysis reaction was carried out in the same manner as in Example 1 for 2 hours. The cumulative current flowing is 12.0Ah, and the average current density is 1000A / m 2 , the liquid temperature in zone 1 is in the range of 15 to 40 °C.
[0122] After the reaction, 332.8 g of composition (B) were obtained in zone 1 . Component analysis of the composition (B) by gas chromatography revealed that 49.7 g of the chlorohydrin ether of the chlorohydrin ether-containing composition (A) was converted to 2-ethylhexyl glycidyl ether without using an alkali agent. Conversion reactions can be carrie...
Embodiment 3
[0125] In addition to adding 12.0 g of sodium chloride and 378.0 g of ion-exchanged water to 210.0 g of composition (A1) to obtain a chlorohydrin ether-containing composition (A) (containing 149.1 g of chlorohydrin ether), and in step 1, The composition (A) containing the chlorohydrin ether was circulated in the area 1 of the electrodialysis apparatus with a pump under stirring, and the electrodialysis reaction was carried out in the same manner as in Example 1 for 2 hours. The cumulative current of the pass is 12.0Ah, and the average current density is 1000A / m 2 , the liquid temperature in zone 1 is in the range of 15 to 40 °C.
[0126] After the reaction, 319.8 g of composition (B) were obtained in zone 1 . Component analysis of the composition (B) by gas chromatography revealed that 54.8 g of the chlorohydrin ether of the chlorohydrin ether-containing composition (A) was converted to 2-ethylhexyl glycidyl ether without using an alkali agent. Conversion reactions can be ca...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com