Synthetic method of bridged metallocene dinuclear catalyst
A synthesis method and metallocene technology, applied in the field of polyolefins, can solve the problems of cumbersome post-processing, low total yield, and many steps, and achieve the effects of avoiding the use of expensive reagents, short synthesis routes, and high yields
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
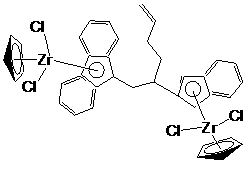
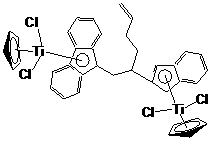
[0029] The preparation method of bridged metallocene dinuclear catalyst is as follows:
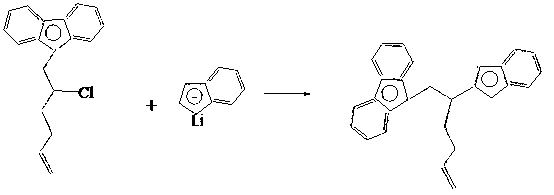
[0030] 1) Synthesis of fluorenyl ligands
[0031] Dissolve 5-chloro-6-bromo-1-hexene in an organic solvent, lower the temperature to -30-0°C, slowly add the fluorene lithium solution dropwise, the dropping time is 1.5-2.5 hours, and continue the reaction for 10-12 hours after the dropping ;
[0032] The molar ratio of 5-chloro-6-bromo-1-hexene to lithium fluorene is: 5-chloro-6-bromo-1-hexene:lithium fluorene=1~1.1:1;
[0033] The organic solvent is selected from anhydrous diethyl ether or petroleum ether.
[0034] The synthesis method is shown in the following formula:
[0035] .
[0036] The choice of solvent is very important. Using anhydrous ether or petroleum ether as a solvent can effectively control the degree of reaction, and can control the reaction of Br in 5-chloro-6-bromo-1-hexene, while Cl does not react. . When using dichloromethane, toluene or tetrahydrofuran and oth...
Embodiment 1
[0058] 1) Synthesis of fluorenyl ligands
[0059] Dissolve 5-chloro-6-bromo-1-hexene in petroleum ether, lower the temperature to -30°C, slowly add fluorene lithium solution dropwise for 2 hours, and continue the reaction for 10 hours after the addition; 5-chloro-6 The molar ratio of bromo-1-hexene to lithium fluorene is: 5-chloro-6-bromo-1-hexene:lithium fluorene=1:1.
[0060] 2) Synthesis of bridged indenylfluorenyl ligands
[0061] Step 1) adding solvent tetrahydrofuran to the obtained fluorenyl ligand, and then reacting with indene lithium to obtain a bridged indenyl fluorenyl ligand. The molar ratio of fluorenyl ligand to indene lithium is 1:1; the reaction temperature is -20°C; the reaction time is 18 hours.
[0062] 3) Synthesis of Ligand Lithium Salt
[0063] Dissolve the ligand obtained in step 2) in n-hexane, add n-butyllithium dropwise, the dropping temperature is -20°C, the molar ratio of ligand to n-butyllithium is 1:2.2, after the addition is completed, the na...
Embodiment 2
[0070] 1) Synthesis of fluorenyl ligands
[0071] Dissolve 5-chloro-6-bromo-1-hexene in petroleum ether, lower the temperature to -20°C, slowly add the fluorene lithium solution dropwise, the dropwise addition time is 2.5 hours, and continue the reaction for 8 hours after the addition; 5-chloro-6 The molar ratio of bromo-1-hexene to lithium fluorene is: 5-chloro-6-bromo-1-hexene:lithium fluorene=1.1:1.
[0072] 2) Synthesis of bridged indenylfluorenyl ligands
[0073] Step 1) adding solvent tetrahydrofuran to the obtained fluorenyl ligand, and then reacting with indene lithium to obtain a bridged indenyl fluorenyl ligand. The molar ratio of fluorenyl ligand to indene lithium is 1:1; the reaction temperature is -10°C; the reaction time is 14 hours.
[0074] 3) Synthesis of Ligand Lithium Salt
[0075] Dissolve the ligand obtained in step 2) in n-hexane, add n-butyllithium dropwise, the dropping temperature is 0°C, the molar ratio of ligand to n-butyllithium is 1:2.5, after t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com