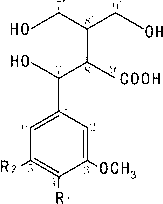
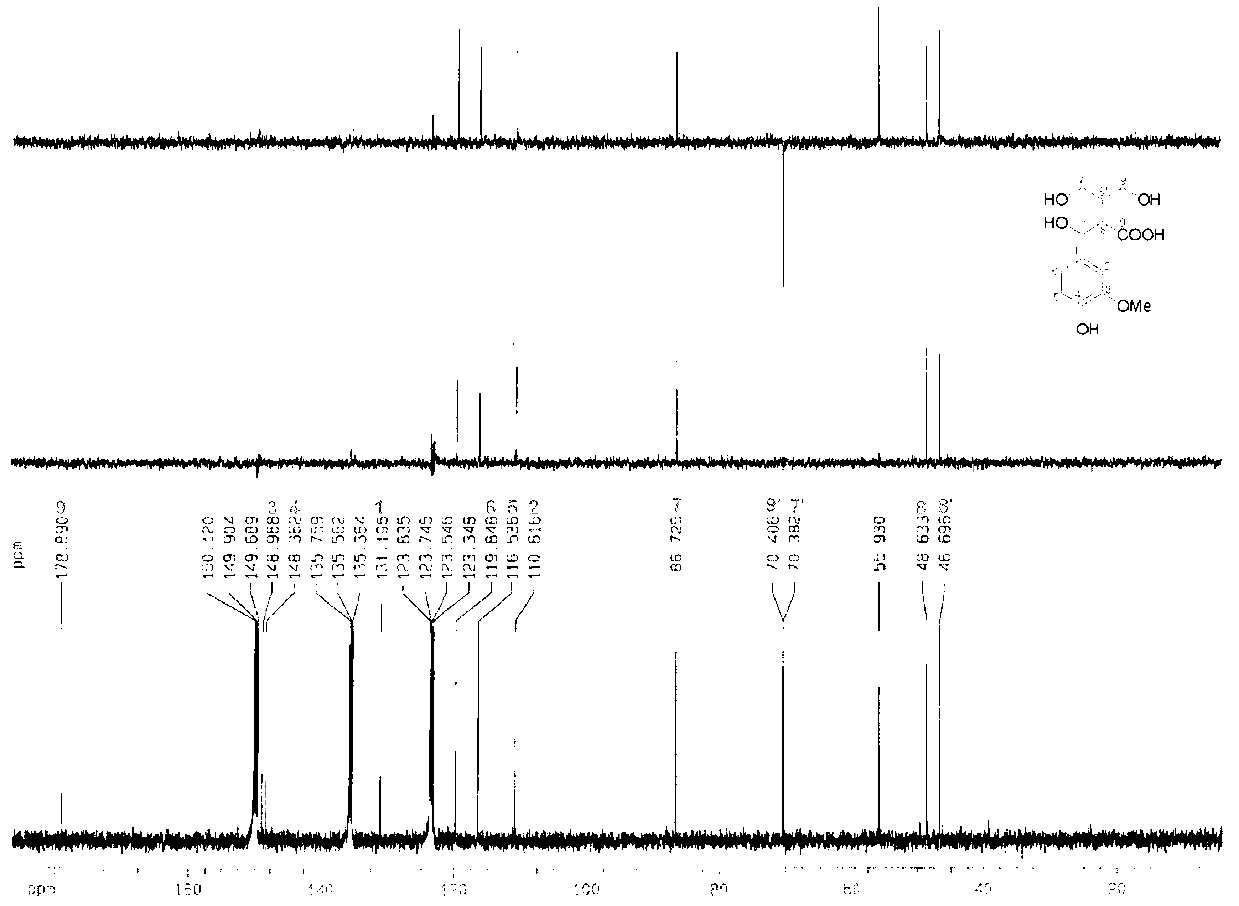
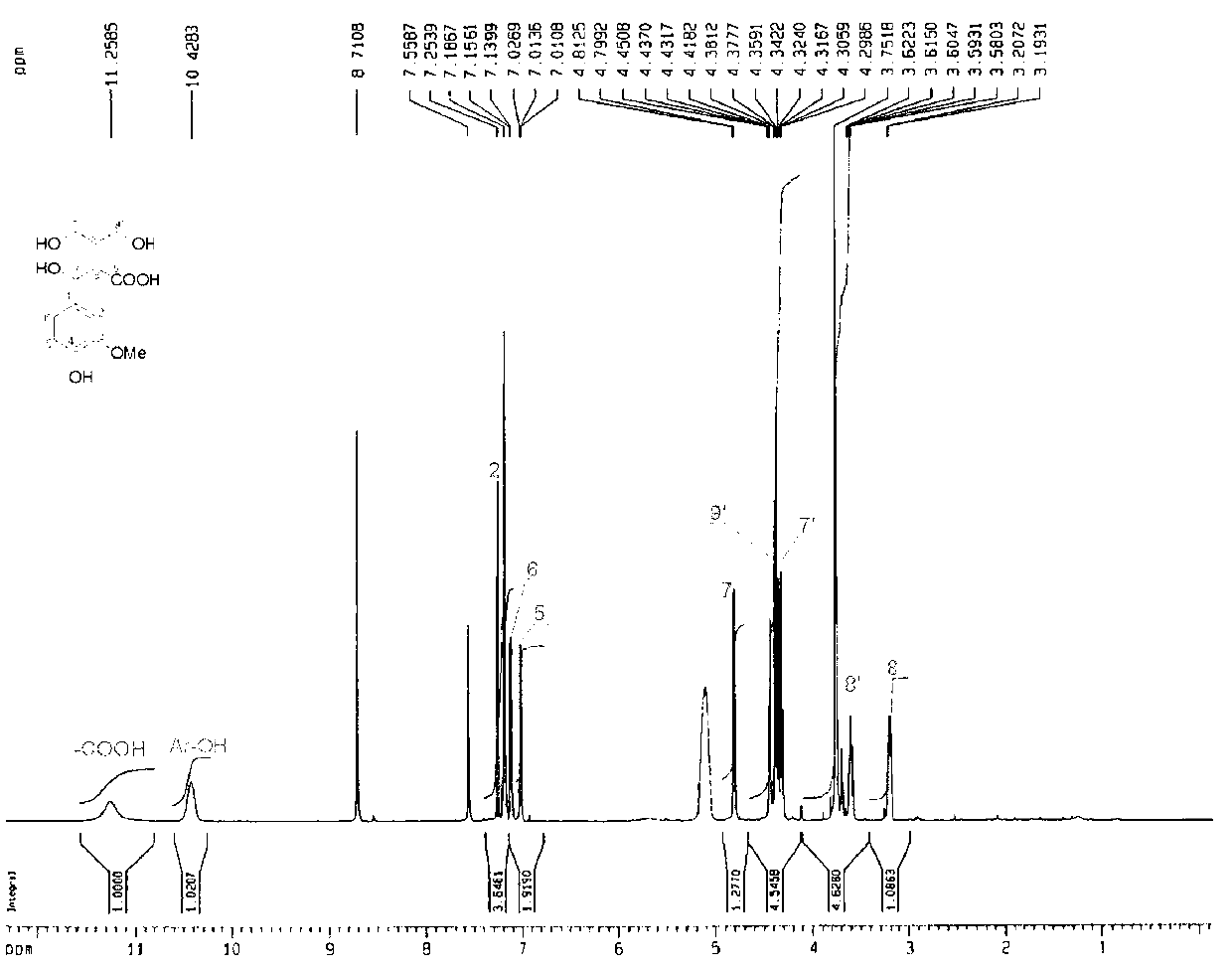
Lignans reduction compounds and preparation method and application thereof
A technology of norlignans and compounds, which is applied in the separation/purification of carboxylic acid compounds, organic chemistry, antiviral agents, etc., can solve the problems of easy changes in the skeleton, complex and unique structures of triterpenoids, etc., and achieve novel structures, active effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] Take 4.5 kg of branches, leaves and / or fruits of Schisandra chinensis, coarsely crush them to 40 meshes, use 70% acetone to ultrasonically extract 5 times, each time for 60 minutes, and combine the extracts; filter the extracts, and concentrate under reduced pressure to 1% of the volume. / 4; stand, filter out the precipitate, and concentrate into 523g extract a; add 784.5g water to extract a, extract 5 times with ethyl acetate equal to the volume of water, combine the extract phases, and concentrate under reduced pressure to 385g Extract b; use 2310g of 200 mesh silica gel to pack the column, add 577.5g of acetone to the extract b to dissolve, then add 385g of 100 mesh silica gel to mix the sample, and put the sample on the column after mixing; the volume ratio is 1:0, 20: 1. Gradient elution with chloroform-methanol organic solvent solution of 9:1, 8:2, 3:2, 1:1, 1:2, 0:1, collecting the gradient eluate, concentrating, monitoring by TLC, combining the same The part, ob...
Embodiment 2
[0048] Take 5 kg of branches, leaves and / or fruits of Schisandra chinensis, coarsely crush them to 20 mesh, use 100% ethanol to ultrasonically extract twice, each time for 50 minutes, and combine the extracts; filter the extracts, concentrate under reduced pressure to 1 / 2 of the volume 3. Stand still, filter out the precipitate, concentrate into 600g of extract a; add 600g of water to extract a, extract 3 times with chloroform equal to the volume of water, combine the extraction phases, concentrate under reduced pressure into 428g of extract b ; Pack the column with 160 mesh silica gel 3.42Kg, add 1284g of acetone to dissolve in the extract b, then add 80 mesh silica gel 342.4g to mix the sample, and put it on the column after mixing the sample; the volume ratio is 1:0, 20:1, 9:1, 8:2, 3:2, 1:1, 1:2, 0:1 gradient elution of n-hexane-acetone organic solvent solution, the gradient eluate was collected, concentrated, monitored by TLC, and the same part; use reversed-phase materia...
Embodiment 3
[0050] Take 6 kg of branches, leaves and / or fruits of Heqing Schisandra, coarsely crush them to 30 mesh, use 80% methanol to ultrasonically extract 4 times, each time for 30 minutes, and combine the extracts; filter the extracts, concentrate under reduced pressure to 1 / 2 of the volume 2. Stand still, filter out the precipitate, concentrate into 635g of extract a; add 1270g of water to extract a, extract 4 times with diethyl ether equal to the volume of water, combine the extraction phases, concentrate under reduced pressure into 500g of extract b ; Use 180 mesh silica gel 3.5Kg to pack the column, add 1000g of acetone to the extract b to dissolve, then add 90 mesh silica gel 600g to mix the sample, and put it on the column after mixing the sample; the volume ratio is 1:0, 20:1, 9 respectively :1, 8:2, 3:2, 1:1, 1:2, 0:1 chloroform-acetone organic solvent solution gradient elution, collect gradient eluate, concentrate, monitor by TLC, merge the same part; Pack the column with r...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com