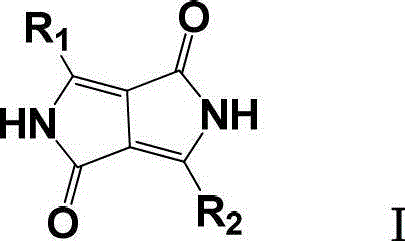
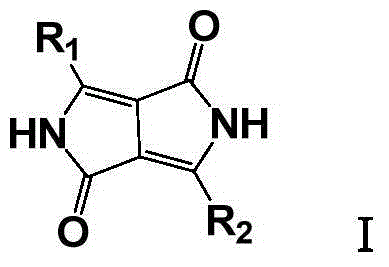
Improved method for preparing pyrrolopyrrole-1,4-diketone derivative
A technology for materials and target substances, applied in the field of preparation of pyrrolopyrrole-1,4-dione derivatives, can solve the problems of inability to separate tert-amyl alcohol from water, complicated treatment process, increased production cost and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] Under nitrogen protection, put tert-amyl alcohol (690g) and sodium tert-amyloxide (230g) in a 2000ml four-neck flask, slowly heat to 110°C, add 4-phenylbenzonitrile (196g, 1.094mol) under stirring, After stirring evenly, di-tert-amyl succinate (183 g, 0.708 mol) was added dropwise, during which the temperature of the reactant was kept at 110-114°C. After the addition was complete, the reaction was kept for 6 hours. Transfer the reactant to a rotary vacuum dryer while it is hot, start the vacuum system to make the vacuum in the dryer reach 1-2mmHg, and then turn on the heating system of the dryer to let the tert-amyl alcohol in the reactant escape and condense The system condenses it to recover tert-amyl alcohol, and a total of tert-amyl alcohol (730g, containing the tert-amyl alcohol generated by the reaction) can be obtained.
[0023] After drying, transfer the dried reactant to a hydrolysis reactor, add methanol / water (2660ml, volume ratio 1:1), raise the temperature ...
Embodiment 2
[0026] Under nitrogen protection, put tert-amyl alcohol (690g) and sodium tert-amyloxide (230g) in a 2000ml four-necked flask, slowly heat to 110°C, add 4-chlorobenzonitrile (151g, 1.098mol) under stirring, and stir After uniformity, di-tert-amyl succinate (183g, 0.708mol) was added dropwise, during which the temperature of the reactant was kept at 110-114°C. After the addition was complete, the reaction was kept for 6h. Transfer the reactant to a rotary vacuum dryer while it is hot, start the vacuum system to make the vacuum in the dryer reach 1-2mmHg, and then turn on the heating system of the dryer to let the tert-amyl alcohol in the reactant escape and condense The system condenses it to recover tert-amyl alcohol, and a total of tert-amyl alcohol (720g, containing the tert-amyl alcohol generated by the reaction) can be obtained.
[0027]After drying, transfer the dried reactant to a hydrolysis reactor, add methanol / water (2660ml, volume ratio 1:1), heat up to 80°C, stir fo...
Embodiment 3
[0030] Under the protection of nitrogen, put tert-amyl alcohol (690g) and sodium tert-amyloxide (230g) into a 2000ml four-neck flask, heat slowly to 110°C, and add 4-tert-butylbenzonitrile (174.2g, 1.094mol) under stirring After stirring evenly, di-tert-amyl succinate (183g, 0.708mol) was added dropwise, during which the temperature of the reactant was kept at 110-114°C. After the addition was complete, the reaction was kept for 6h. Transfer the reactant to a rotary vacuum dryer while it is hot, start the vacuum system to make the vacuum in the dryer reach 1-2mmHg, and then turn on the heating system of the dryer to let the tert-amyl alcohol in the reactant escape and condense This was condensed by the system to recover tert-amyl alcohol, and a total of tert-amyl alcohol was obtained (725 g).
[0031] After drying, transfer the dried reactant to a hydrolysis reactor, add methanol / water (2660ml, volume ratio 1:1), heat up to 80°C, stir for 1h, adjust the pH to neutral with conc...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com