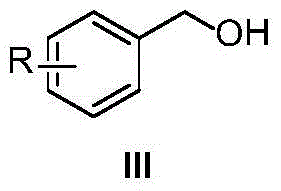
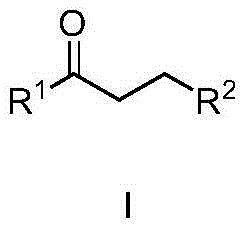
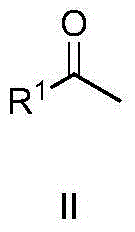
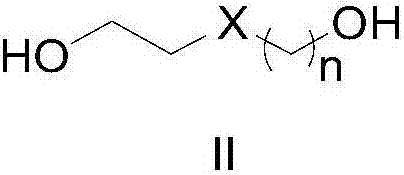
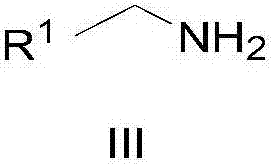
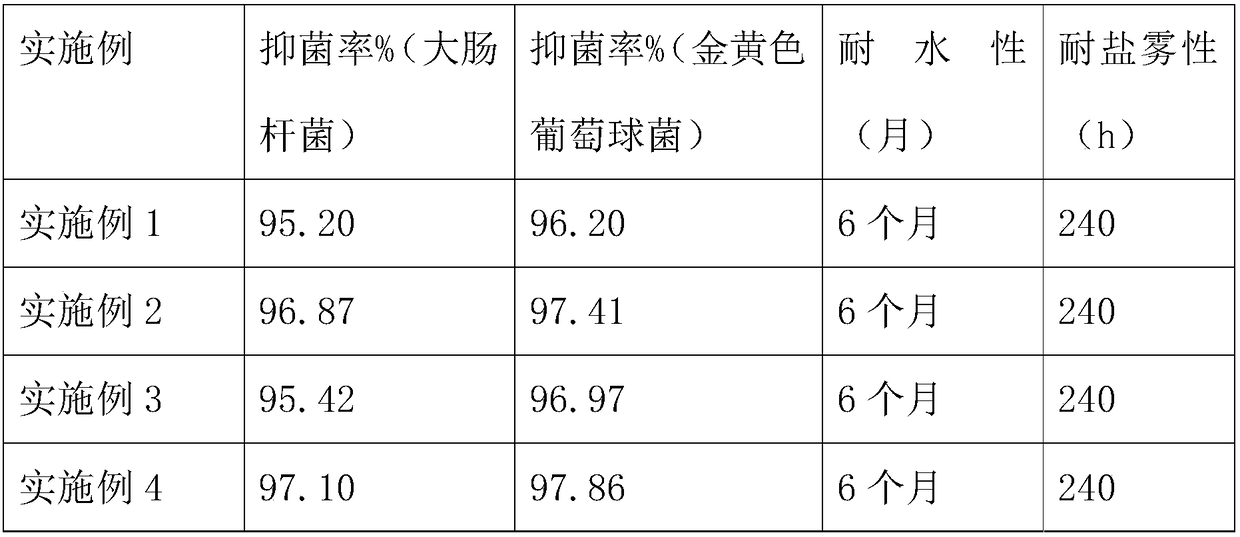
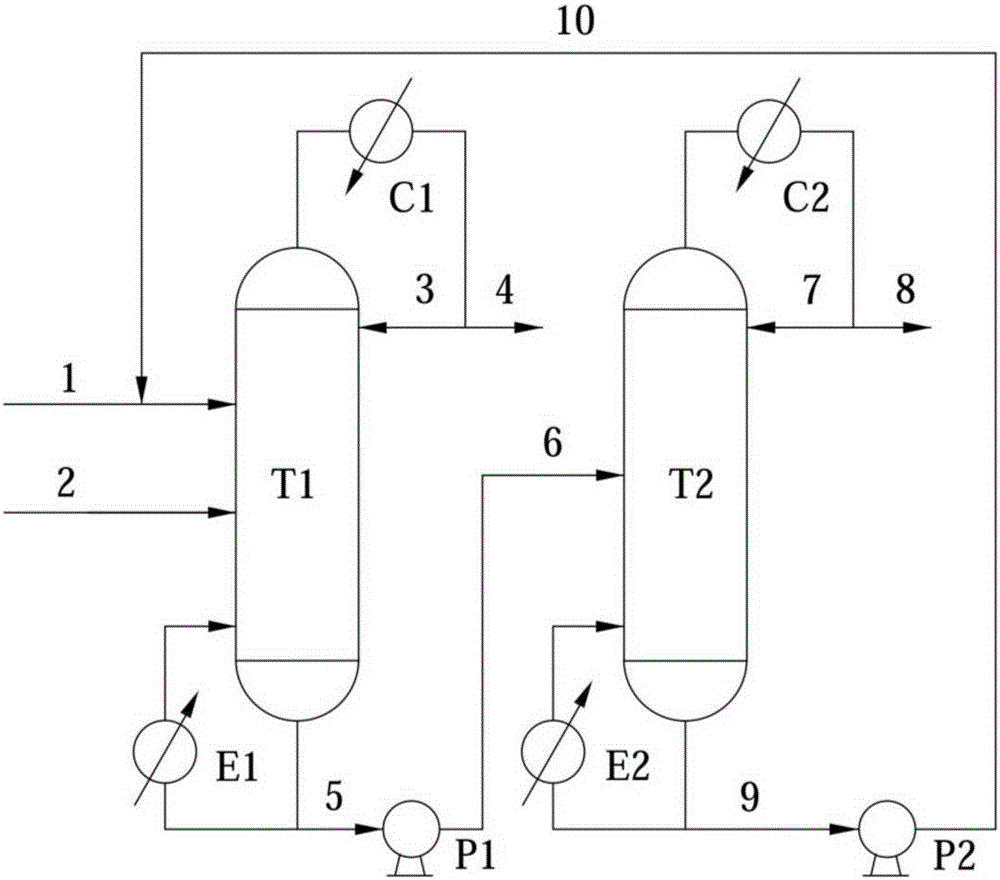
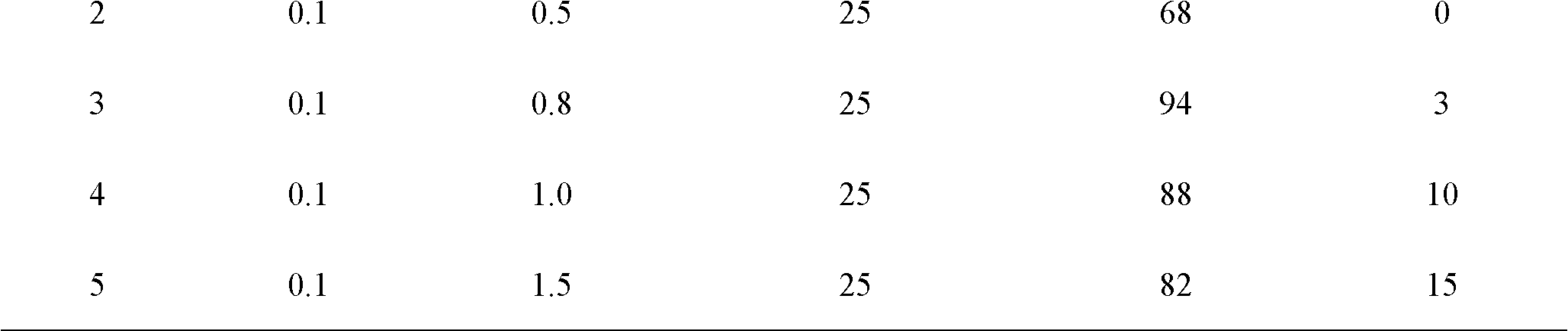Patents
Literature
73 results about "Tert-Amyl alcohol" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
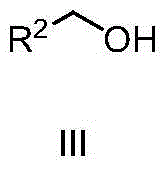
Tert-Amyl alcohol (TAA), systematic name 2-methylbutan-2-ol (2M2B), is a branched pentanol. Historically TAA has been used an anesthetic and more recently it has also been used as a recreational drug similar to ethanol because TAA is mostly a positive allosteric modulator for GABAA receptors in the same way as ethanol. This means that TAA causes calming effects within the central nervous system by interacting indirectly (allosterically) with GABAA receptors and enhances (positive effect) their activity.
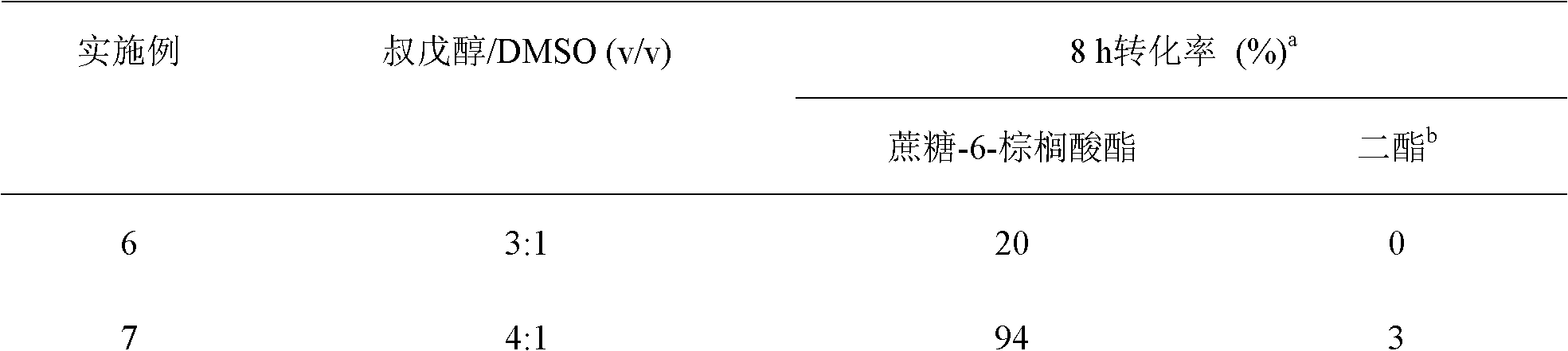
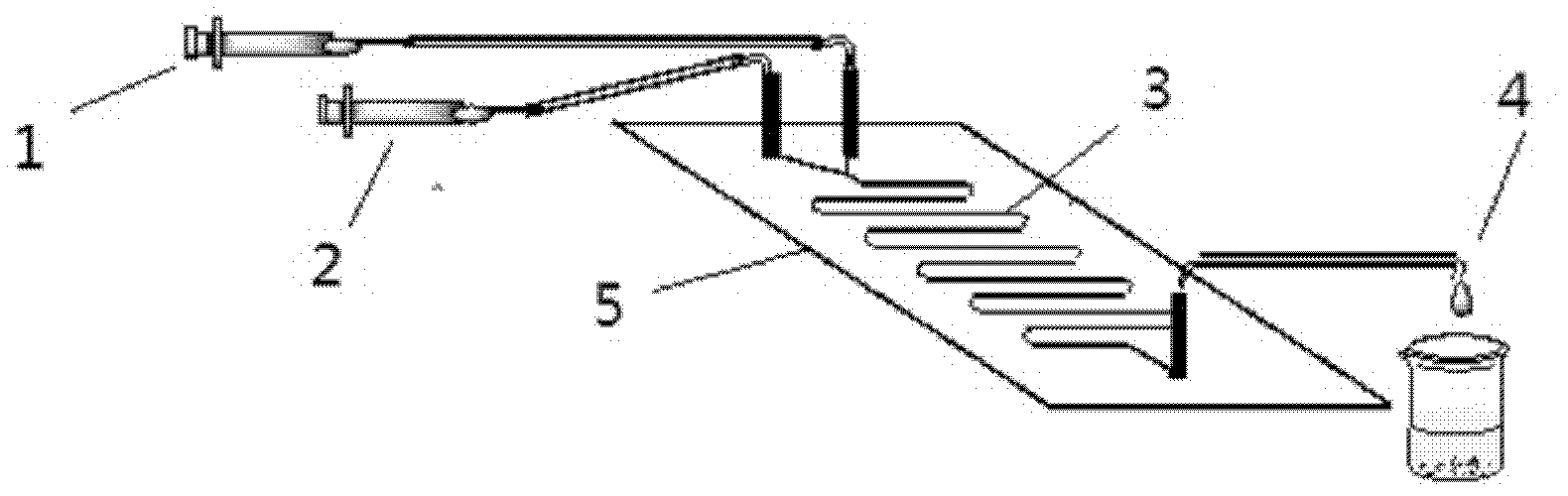
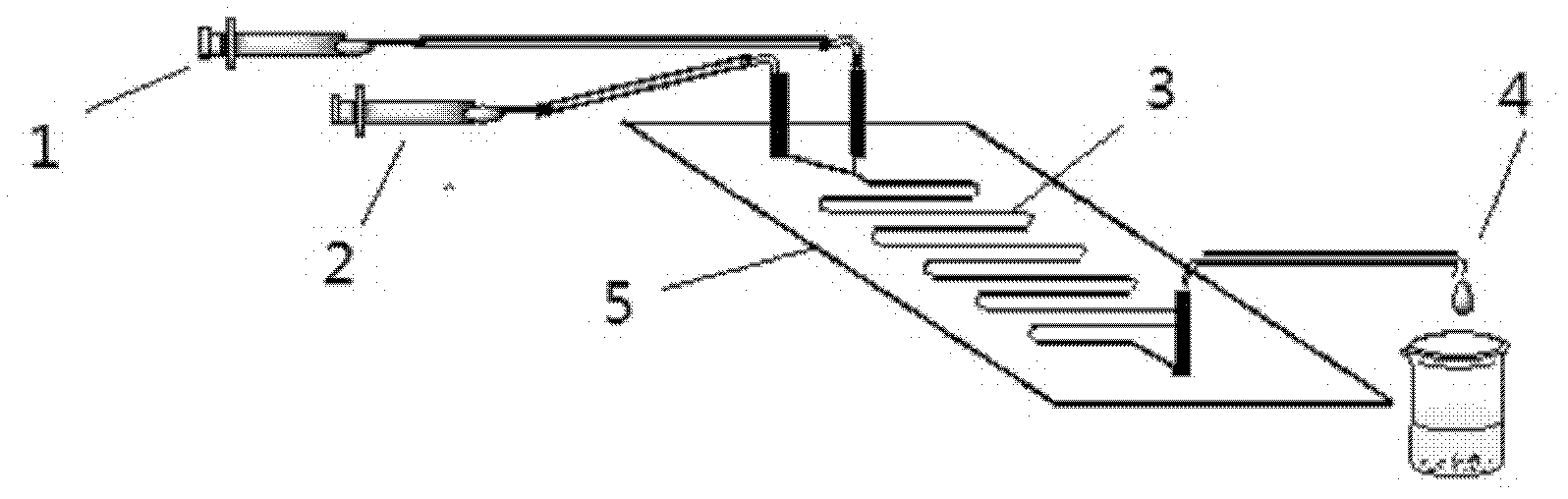
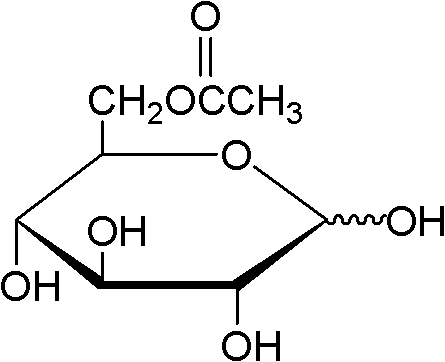
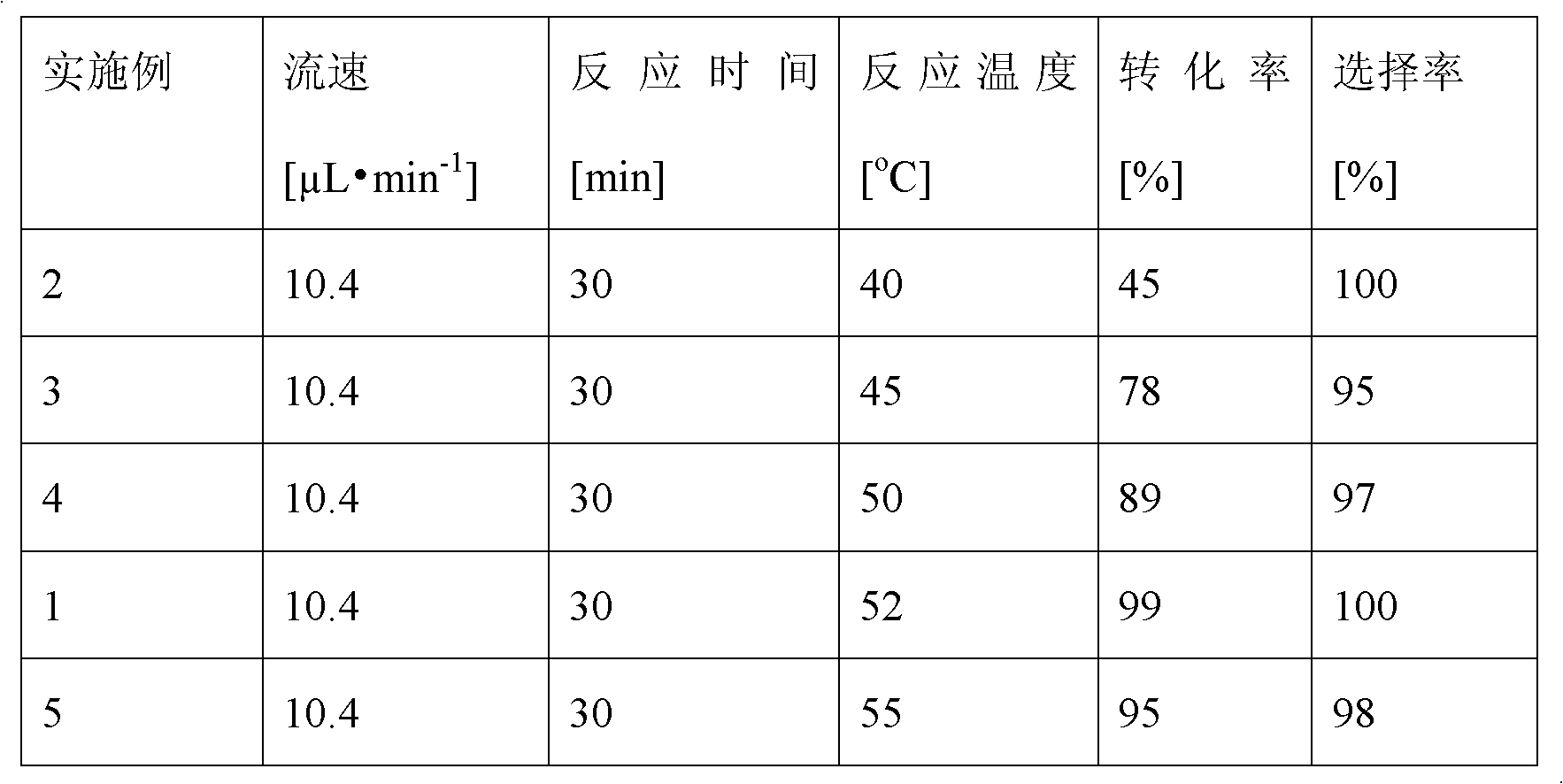
Method for on-line synthesizing sucrose-6-acetate catalyzed by lipase
ActiveCN103184257AReduce usageShort reaction timeBioreactor/fermenter combinationsBiological substance pretreatmentsAcetic acidMicrofluidic channel
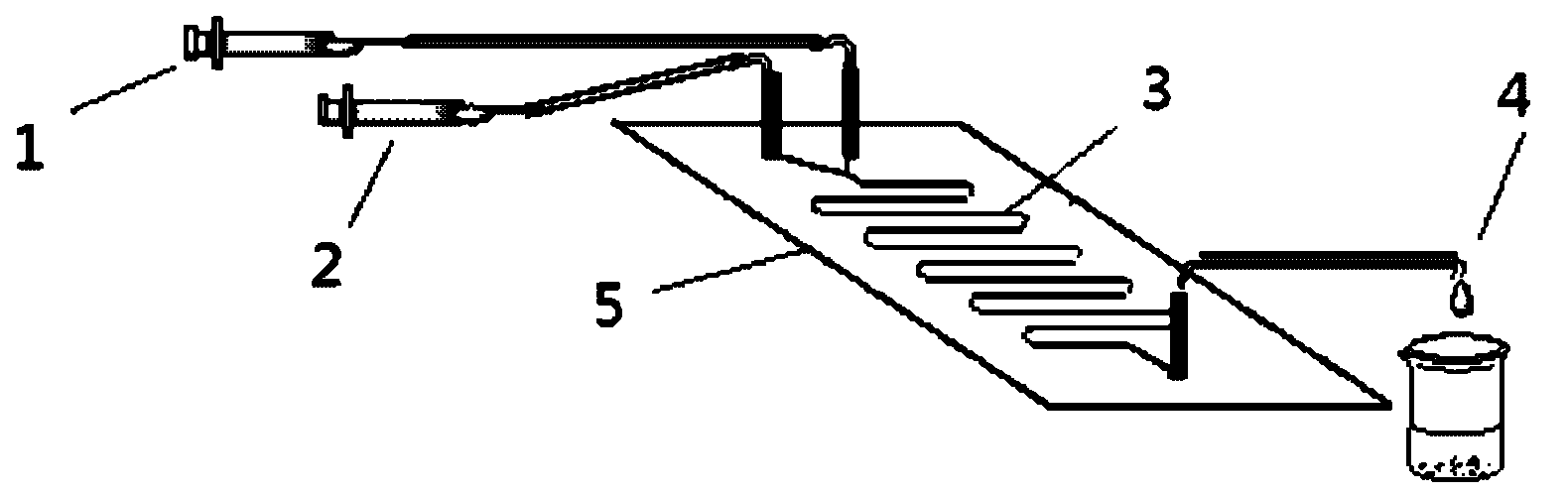
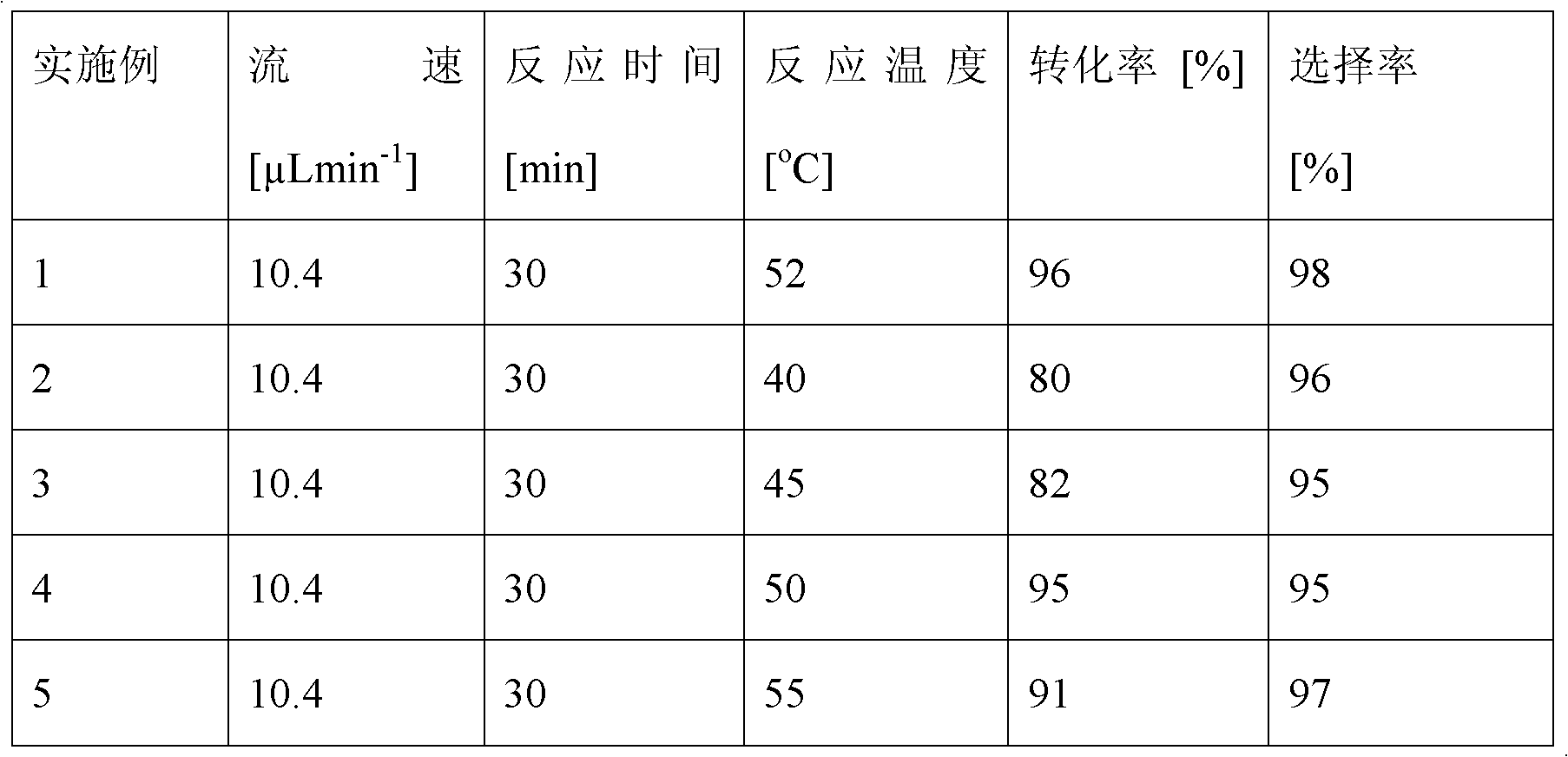
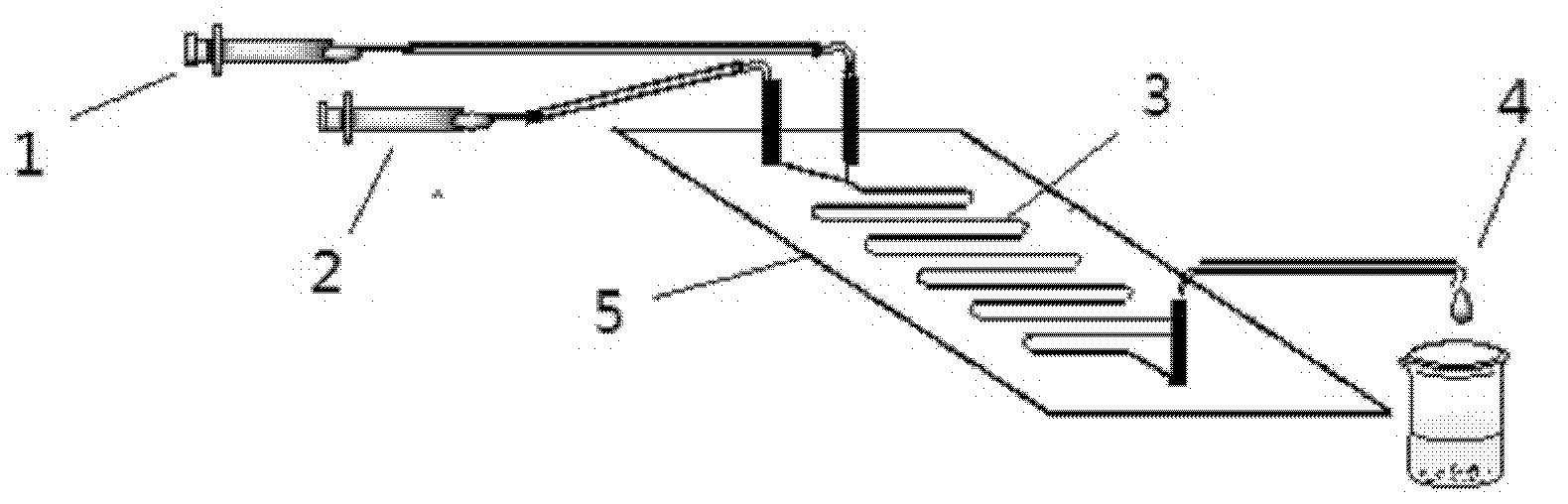
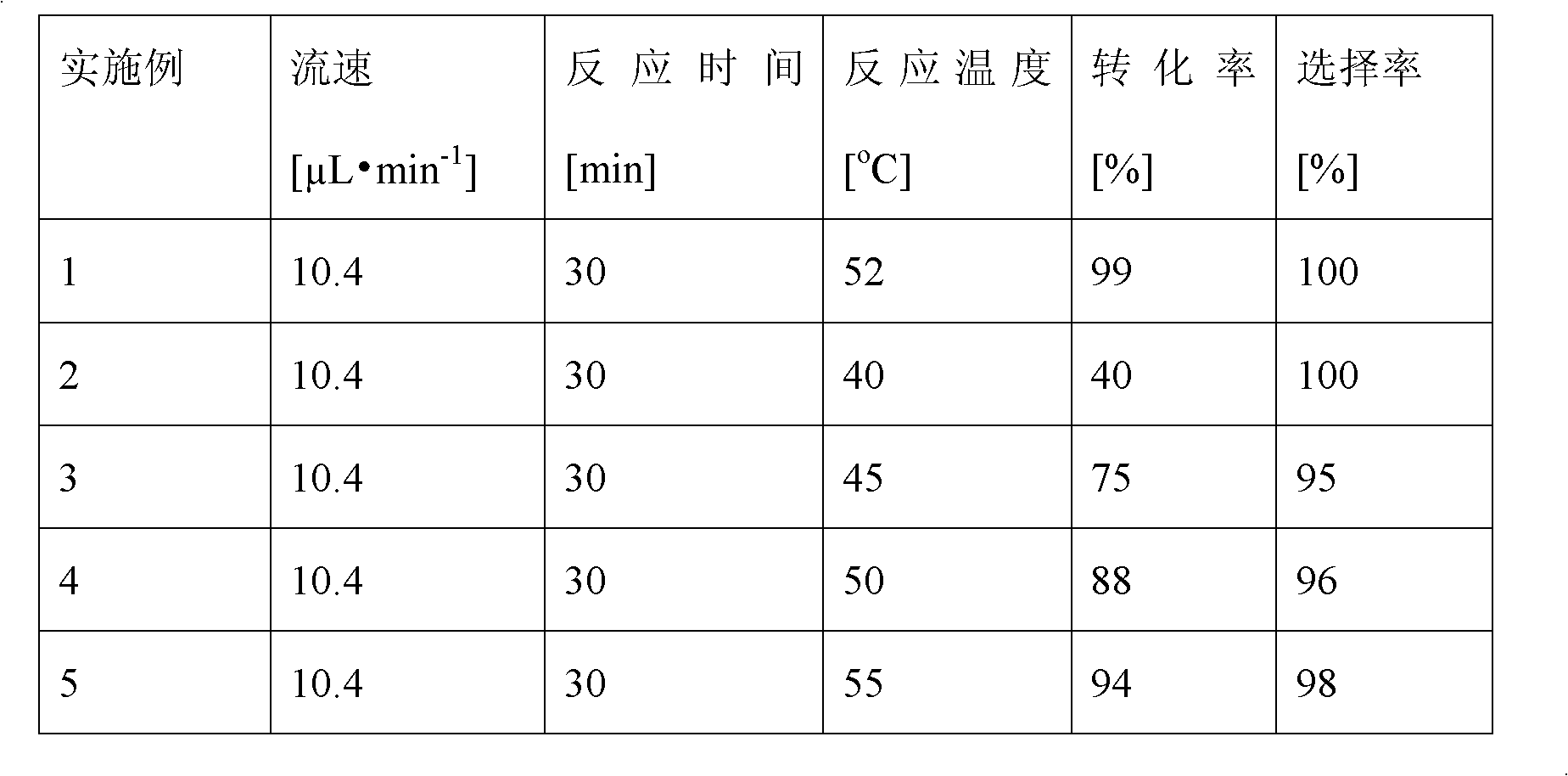
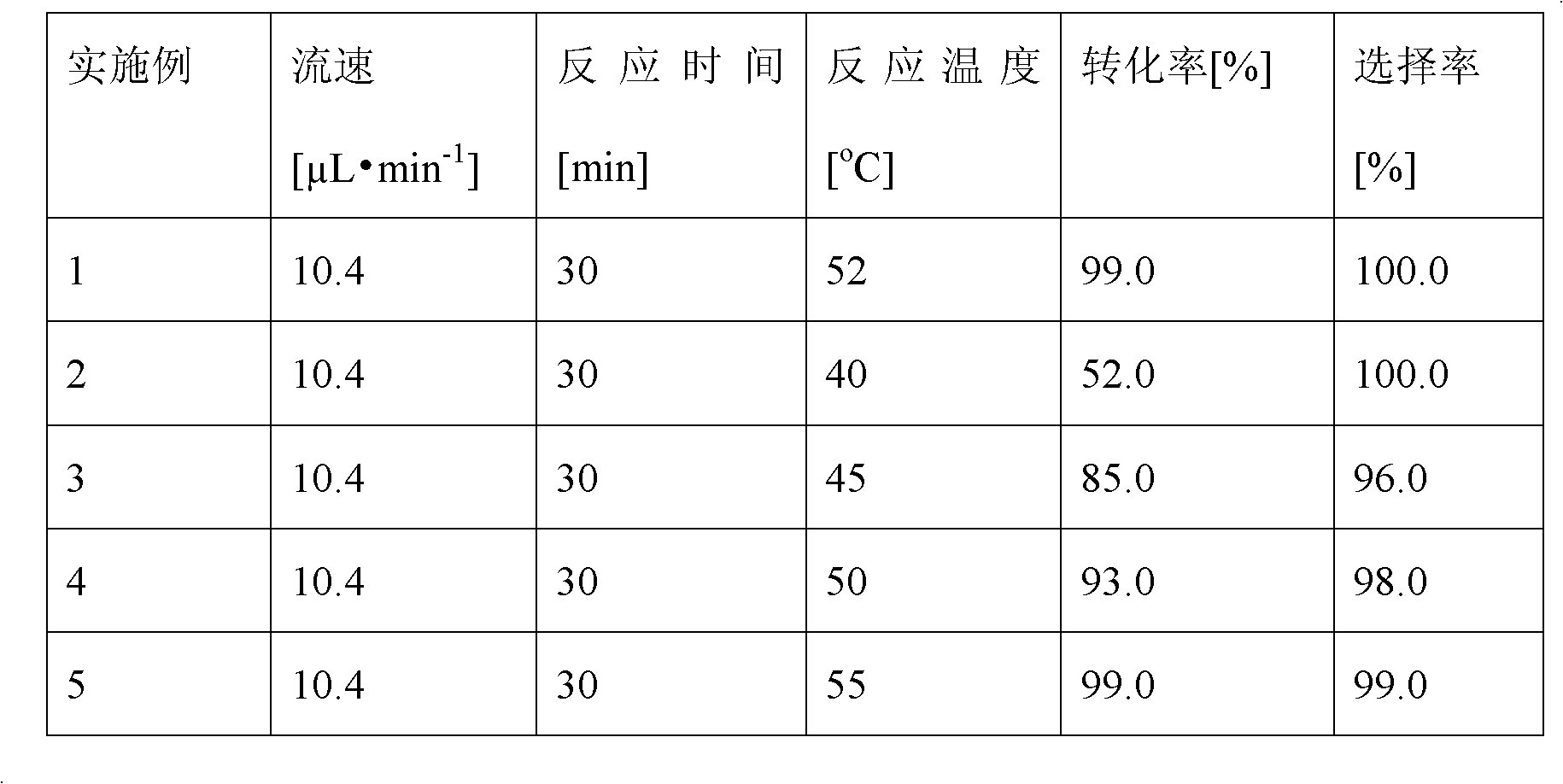
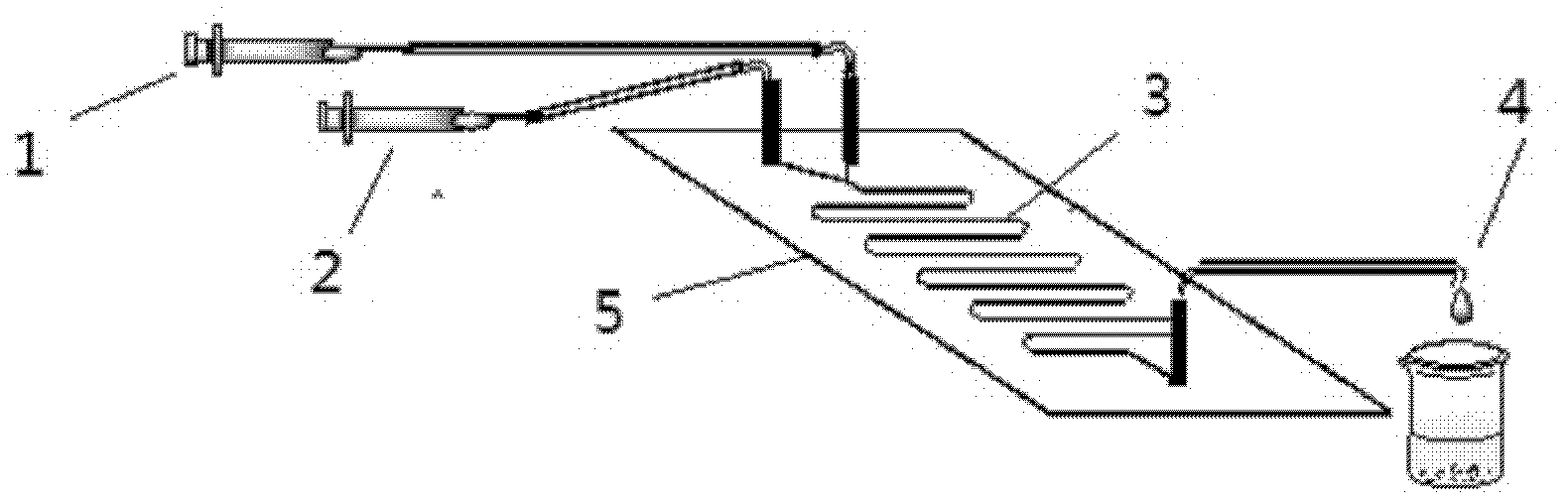
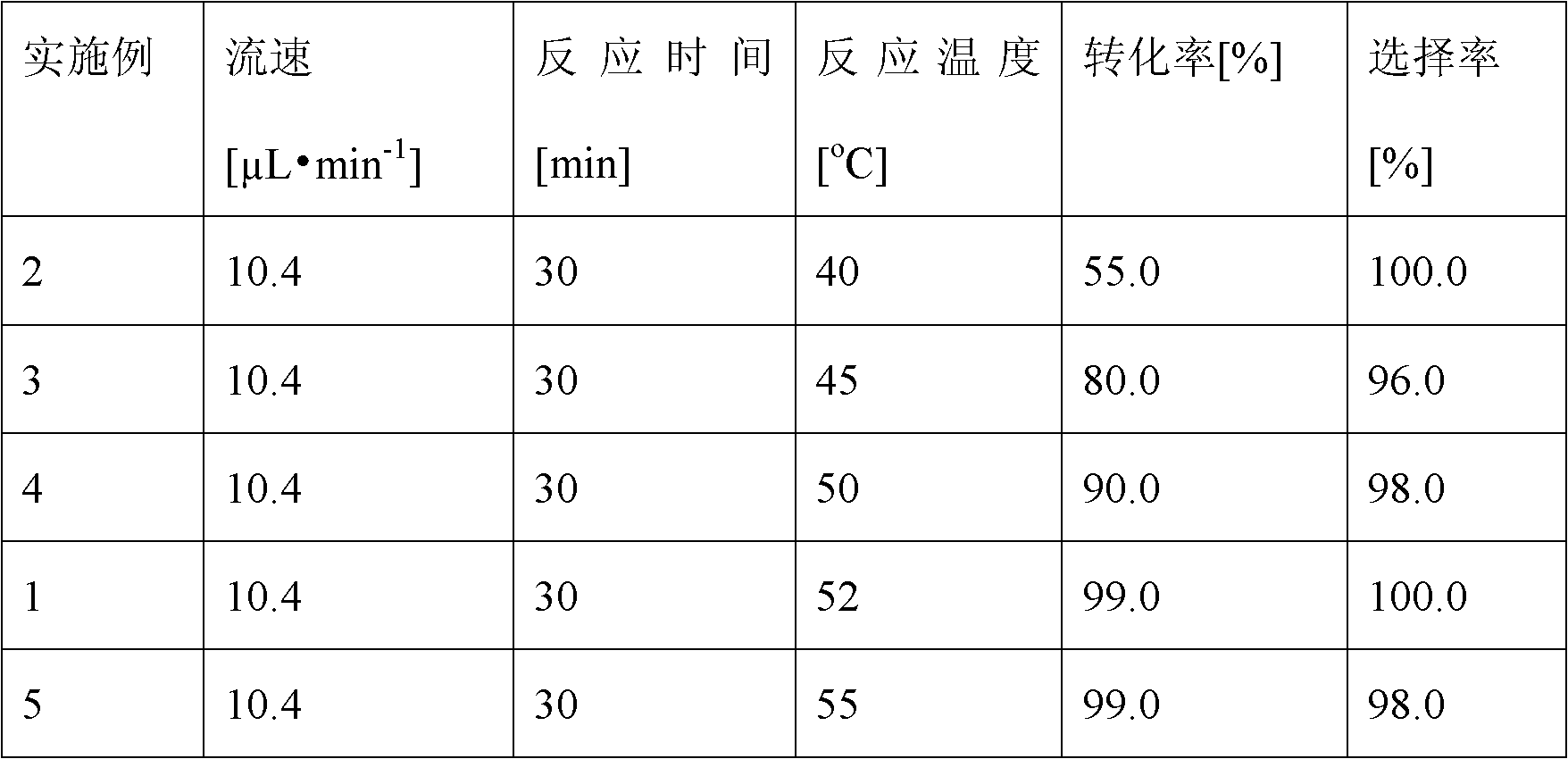
The invention discloses a method for on-line synthesizing sucrose-6-acetate catalyzed by lipase. According to the method, sucrose and vinyl acetate with a molar ratio being 1 : 15-20 are used as raw materials; 0.5-1.0 g of the lipase (Lipozyme TLIM) is used as a catalyst and a mixed solvent formed by tert-amyl alcohol and DMSO is used as a reaction solvent. The lipase (Lipozyme TLIM) is uniformly filled in a reaction channel of a microfluidic channel reactor, wherein the inner diameter of the reaction channel of the microfluidic channel reactor is 0.8-2.4 mm, and the length of the reaction channel is 0.5-1.0 m; the raw materials and the reaction solvent are continuously introduced into the reaction channel for an acylation reaction, wherein the temperature of the acylation reaction is controlled at 40-55 DEG C and the time of the acylation reaction is 20-40 min; a reaction liquid is collected on-line; and the sucrose-6-acetate is obtained after the reaction liquid is subjected to conventional post-treatment. The method provided by the invention has the advantages of short reaction time, high selectivity and high yield.
Owner:ZHEJIANG UNIV OF TECH
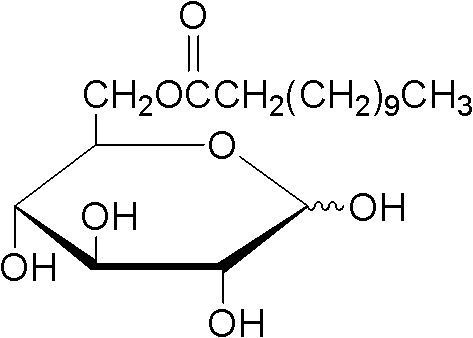
Method for synthesizing sucrose-6-palmitate by using lipase through catalytic selectivity
ActiveCN102161683AImprove conversion rateHigh selectivityEsterified saccharide compoundsSugar derivativesSaccharophagus degradansPalmitates
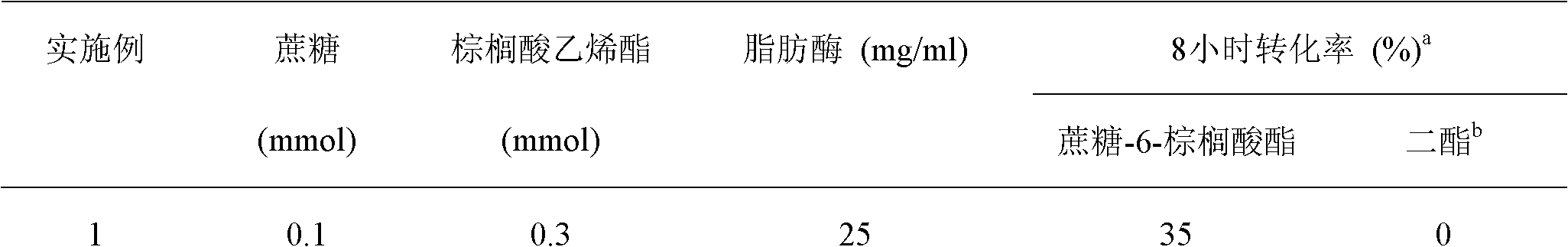
The invention discloses a method for synthesizing sucrose-6-palmitate by using lipase through catalytic selectivity. The sucrose-6-palmitate is prepared by taking sucrose and palmitic acid vinyl ester as raw materials, the lipase, namely Lipozyme RM IM as a catalyst, and mixed solvent of tert-amyl alcohol and dimethylsulfoxide (DMSO) in a volume ratio of 4:1 as reaction medium through an acylation reaction. By the method, sucrose palmitate which is highly monoesterified is synthesized at high yield, and reaction time is greatly shortened.
Owner:ZHEJIANG UNIV OF TECH
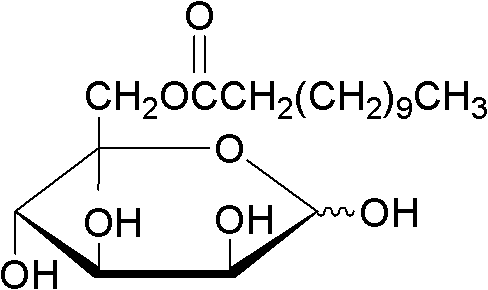
Method of using lipase to catalyze and synthesize glucose-6-laurate on line
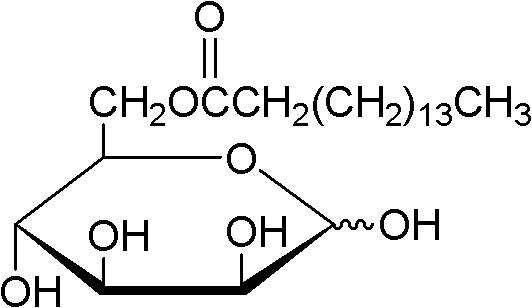
The invention discloses a method of using lipase to catalyze and synthesize glucose-6-laurate on line. The method comprises the following steps: taking glucose and vinyl laurate as raw materials according to molar ratio of 1:8-12, taking 0.5-1.0 g of lipase Lipozyme TLIM as a catalyst, taking tert-amyl alcohol and DMSO (dimethyl sulfoxide) mixed solvent as a reaction solvent, uniformly filling the lipase Lipozyme TLIM into a reaction channel of a microfluidic channel reactor, wherein the inner diameter of the reaction channel of the microfluidic channel reactor is 0.8-2.4 mm, and the reaction channel is 0.5-1.0 m long, continuously introducing the raw materials and the reaction solvent into the reaction channel to perform acylation reaction, controlling the temperature of acylation reaction to be 40-55 DEG C, and keeping the acylation reaction for 15-35 min, on line collecting reaction liquid, and conventionally post-processing the reaction liquid to obtain glucose-6-laurate. The method has the advantages of short reaction time, high selectivity and high yield.
Owner:ZHEJIANG UNIV OF TECH
Method of using lipase to catalyze and synthesize mannose-6-laurate on line
The invention discloses a method of using lipase to catalyze and synthesize mannose-6-laurate on line. The method comprises the following steps: taking mannose and vinyl laurate as raw materials according to molar ratio of 1:8-12, taking 0.5-1.0 g of lipase Lipozyme TLIM as a catalyst, taking tert-amyl alcohol and DMSO (dimethyl sulfoxide) mixed solvent as a reaction solvent, uniformly filling the lipase Lipozyme TLIM into a reaction channel of a microfluidic channel reactor, wherein the inner diameter of the reaction channel of the microfluidic channel reactor is 0.8-2.4 mm, and the reaction channel is 0.5-1.0 m long, continuously introducing the raw materials and the reaction solvent into the reaction channel to perform acylation reaction, controlling the temperature of acylation reaction to be 40-55 DEG C, and keeping the acylation reaction for 15-35 min, on line collecting reaction liquid, and conventionally post-processing the reaction liquid to obtain mannose-6-laurate. The method has the advantages of short reaction time, high selectivity and high yield.
Owner:ZHEJIANG UNIV OF TECH
Method for on-line synthesizing glucose-6-acetate catalyzed by lipase
The invention discloses a method for on-line synthesizing glucose-6-acetate catalyzed by lipase. According to the method, glucose and vinyl acetate with a molar ratio being 1 : 16-22 are used as raw materials; 0.5-1.0 g of the lipase (Lipozyme TLIM) is used as a catalyst and a mixed solvent formed by tert-amyl alcohol and DMSO is used as a reaction solvent. The lipase (Lipozyme TLIM) is uniformly filled in a reaction channel of a microfluidic channel reactor, wherein the inner diameter of the reaction channel of the microfluidic channel reactor is 0.8-2.4 mm, and the length of the reaction channel is 0.5-1.0 m; the raw materials and the reaction solvent are continuously introduced into the reaction channel for an acylation reaction, wherein the temperature of the acylation reaction is controlled at 40-55 DEG C and the time of the acylation reaction is 15-35 min; a reaction liquid is collected on-line; and the glucose-6-acetate is obtained after the reaction liquid is subjected to conventional post-treatment. The method provided by the invention has the advantages of short reaction time, high selectivity and high yield.
Owner:ZHEJIANG UNIV OF TECH
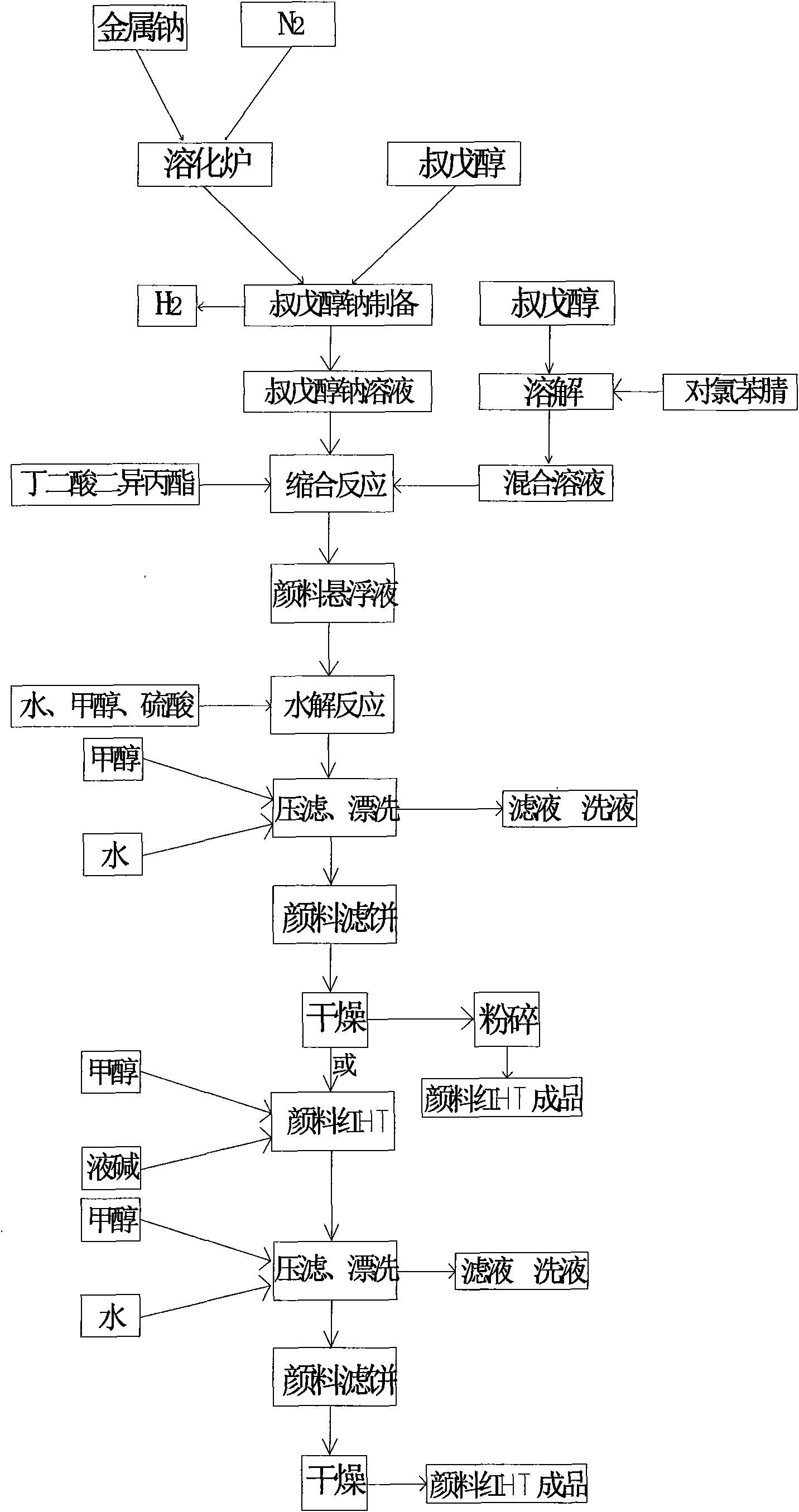
Production process of pigment red HT
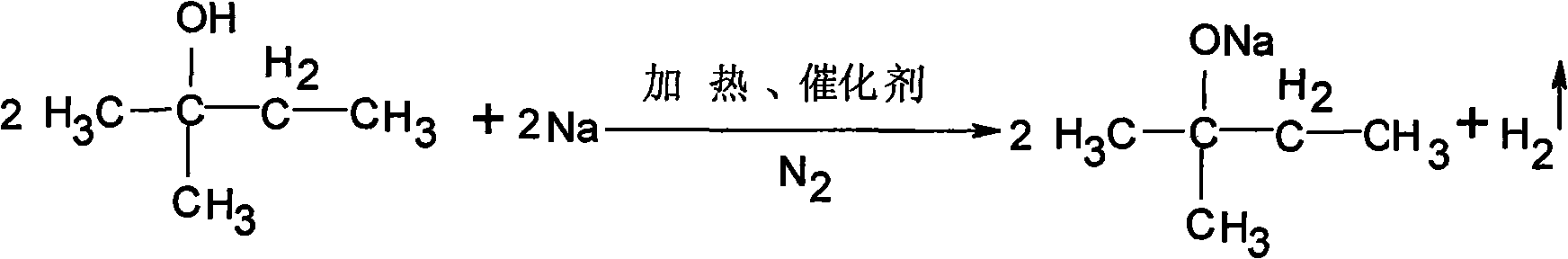
The invention relates to a production process of pigment red HT, comprising steps of redox reaction, condensation reaction and hydrolysis reaction. The redox reaction step is realized by gradually adding 90-110ml of ferric trichloride tert-amyl alcohol solution with a concentration of 5 percent as a catalyst, introducing N2 at a temperature of 115-122 DEG C, and maintaining redox reaction for one hour to obtain tert-amyl alcohol sodium; the hydrolysis reaction step is realized by adding 180-200kg of concentrated sulfuric acid with a mass concentration of 96 percent and hydrolyzing the concentrated sulfuric acid for 2-4 hours at a temperature of 40-60 DEG C, filtering, rinsing, drying and breaking to obtain the product. The invention has the advantages of short technique process, high efficiency and little side product; and the prepared pigment red HT has extremely fine particles and high transparency and is bright in color.
Owner:南通市埃唯卡新材料有限公司
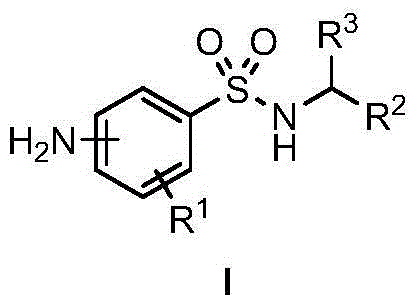
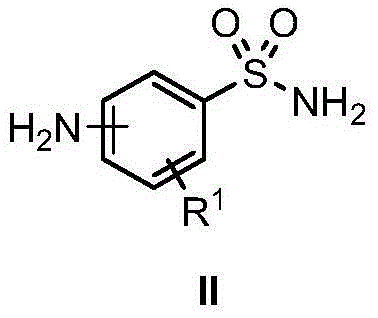
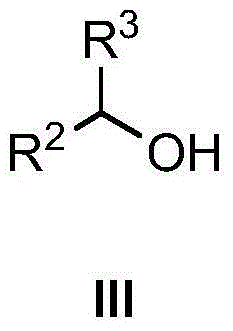
Amino-(N-alkyl) benzsulfamide synthesis method
InactiveCN106146358AImprove economyMeet the requirements of green chemistrySulfonic acid amide preparationMetallocenesSynthesis methodsEvaporation
The invention discloses an amino-(N-alkyl) benzsulfamide synthesis method. The method comprises the steps of adding aminobenzenesul fonamide, an iridium complex catalyst, alkali, compound alcohol and the solvent tert-amyl alcohol into a reaction container for reaction lasting several hours at 120-150 DEG C, then reducing temperature to room temperature, conducting rotary evaporation to remove the solvent, and then conducting column separation to obtain the target compound. Commercial aminobenzenesul fonamide and nearly non-toxic compound alcohol are used as starting materials, only water is generated as reaction byproduct, and no environment harm is done; reaction atom economy is high.
Owner:NANJING UNIV OF SCI & TECH
Microfluidic channel reactor and application thereof in synthesis of sucrose-6-palmitate
ActiveCN103182277AReduce usageShort reaction timeEsterified saccharide compoundsSugar derivativesSucrosePalmitates
The invention discloses a method for on-line synthesizing sucrose-6-palmitate catalyzed by a lipase. According to the method, sucrose and vinyl palmitate with a molar ratio being 1 : 3-8 are used as raw materials; 0.5-0.8 g of the lipase (Lipozyme TLIM) lipase is used as a catalyst and a mixed solvent formed by tert-amyl alcohol and DMSO is used as a reaction solvent. The lipase (Lipozyme TLIM) is uniformly filled in a reaction channel of a microfluidic channel reactor, wherein the inner diameter of the reaction channel of the microfluidic channel reactor is 0.8-2.4 mm, the length of the reaction channel is 0.5-1.0 m; the raw materials and the reaction solvent are continuously introduced into the reaction channel driven by an injection pump for an acylation reaction, wherein a temperature of the acylation reaction is controlled at 40-55 DEG C and the time of the acylation reaction is 20-40 min; a reaction liquid is collected on-line; and the sucrose-6-palmitate is obtained after the reaction liquid is subjected to conventional post-treatment. The method provided by the invention has the advantages of short reaction time, high selectivity and high yield.
Owner:ZHEJIANG UNIV OF TECH
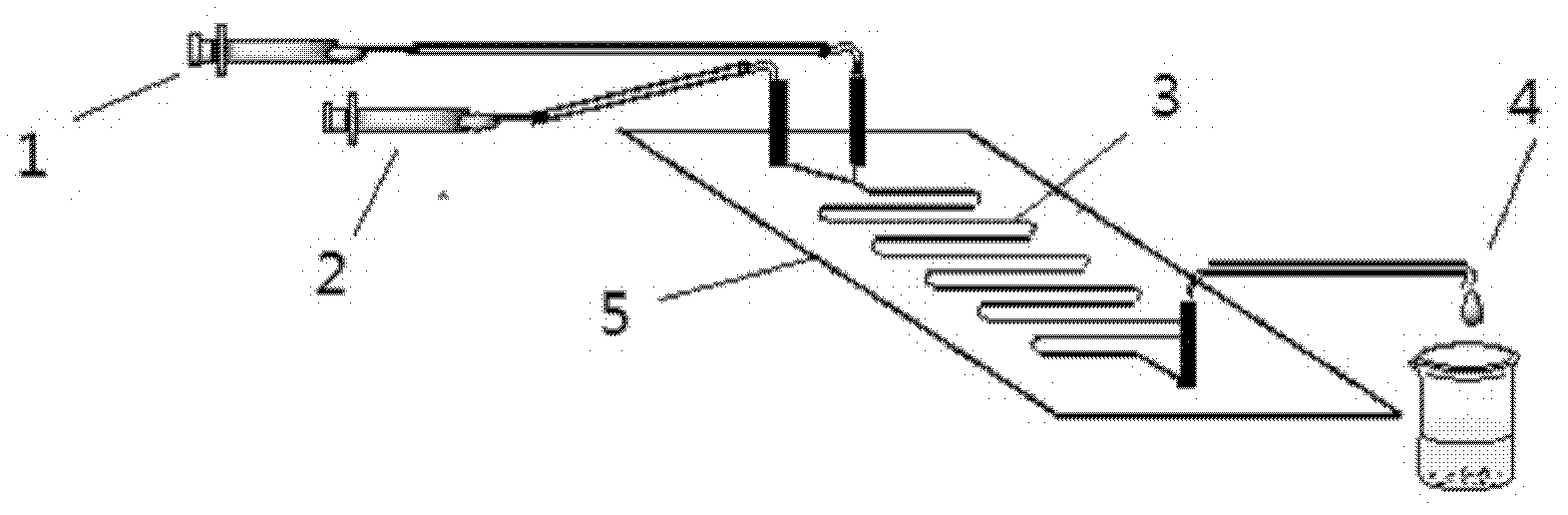
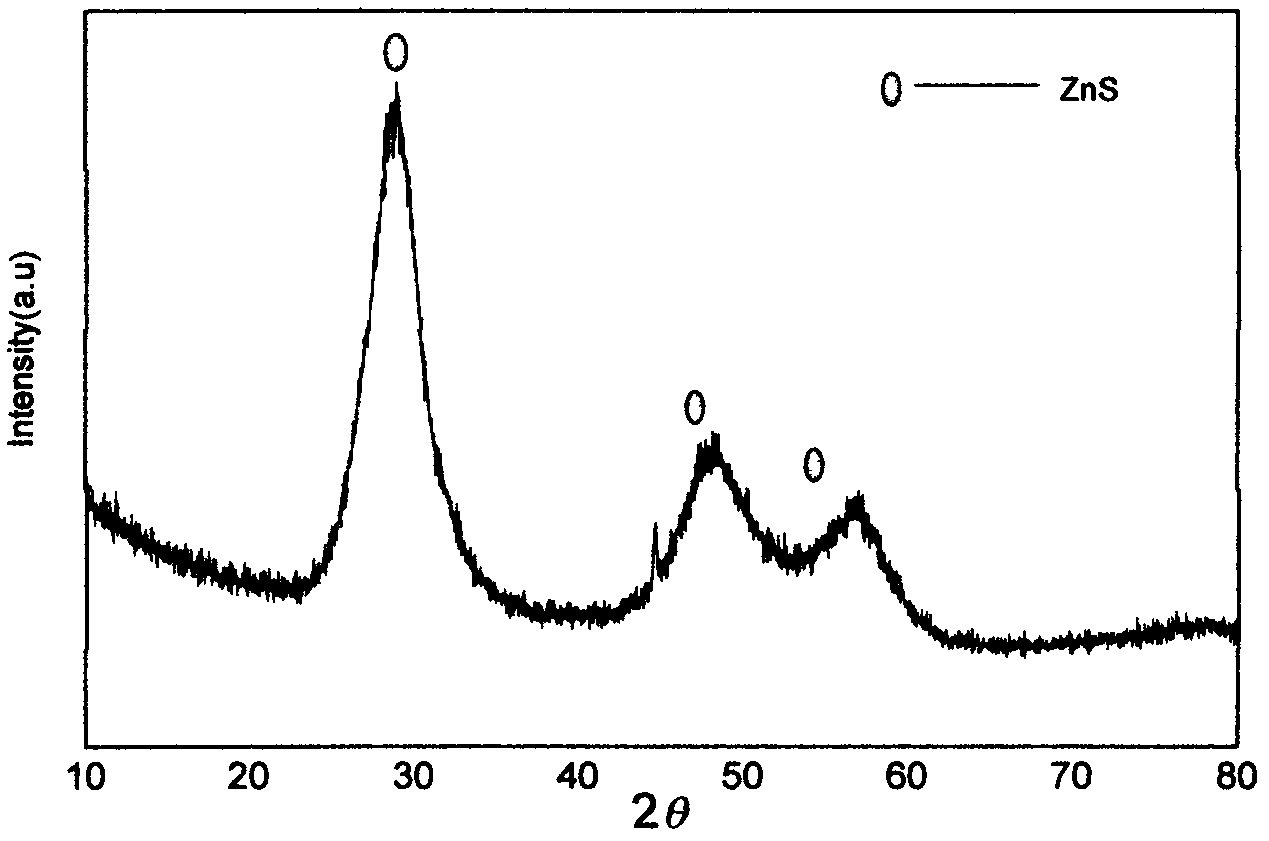
Low-temperature mechanical alloying method for preparing sulphur selenide submicro powder material
InactiveCN102616753AAvoid introducingReaction raw materials are readily availableSelenium/tellurium compundsIsobutanolEthylenediamine
The invention discloses a low-temperature mechanical alloying method for preparing a sulphur selenide submicro powder material. Simple substance metal powder and simple substance non-metal powder are added into a ball mill tank and ball milled according to rated ball material ratio and set rotating speed with alcohol and amine mixing liquid serving as a process control agent, and ball mill products are washed centrifugally and dried to obtain target products. The simple substance metal powder is one of Zn powder, Sn powder and Cu powder. The simple substance non-metal powder is S powder or Sepowder. The process control agent is mixture of alcohol and amine with volume ratio as 1-20:1, the alcohol is one of ethanol, glycol, n-butyl alcohol, isobutanol, isoamylol, tert-amyl alcohol and glycerol, and the amine is one of ethylenediamine, iso-butylamine, diisopropylamine, hexamethylenediamine and triethylamine. The raw material is easy to obtain, products are pure, energy consumption is low, product appearance is easy to control, technology is simple, and the method is suitable for industrial production.
Owner:GUILIN UNIVERSITY OF TECHNOLOGY
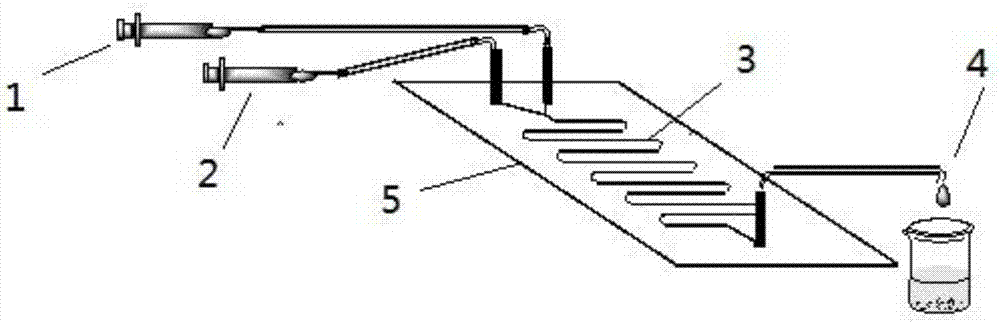
Method for online synthesizing 5'-O-palmitoyl uridine in lipozyme catalysis mode
ActiveCN105838600AShort reaction timeImprove conversion rateEnzyme production/based bioreactorsFermentationSolventPalmitic acid
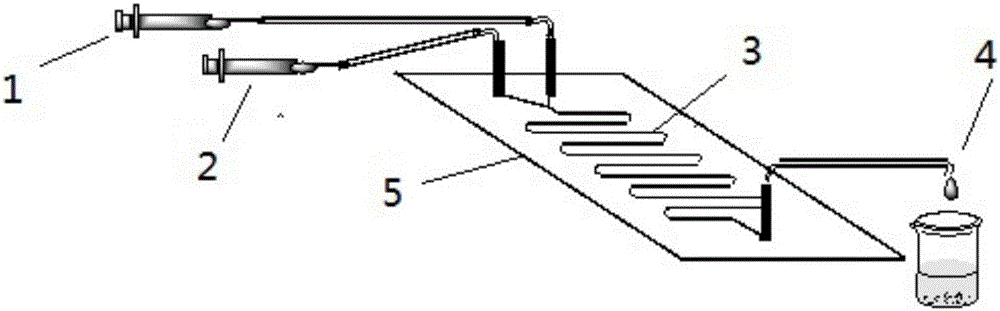
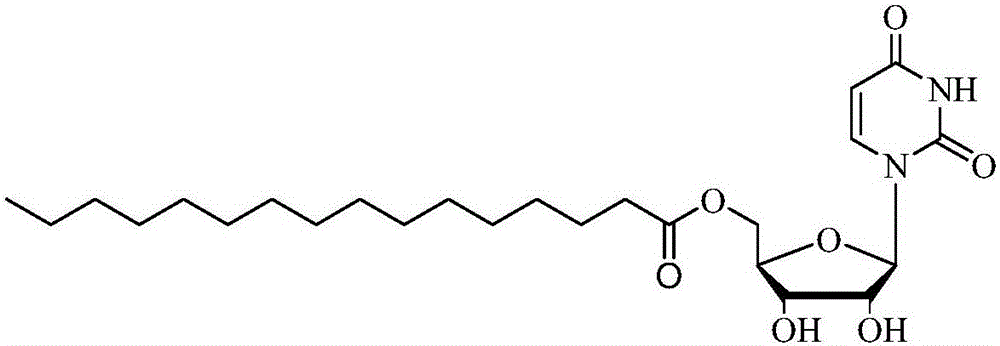
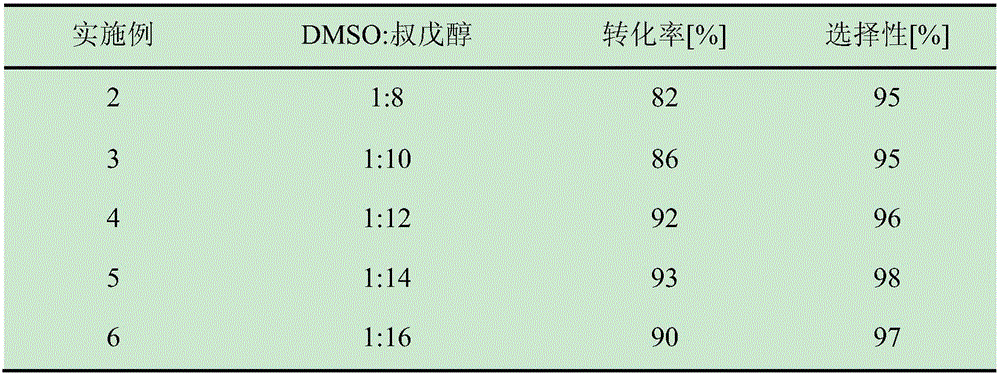
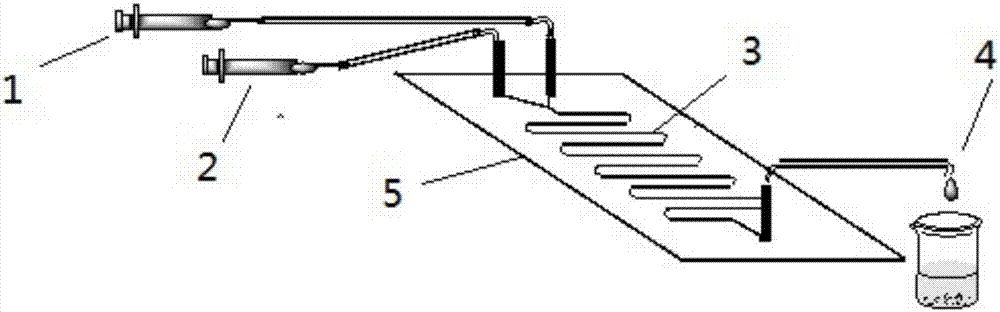
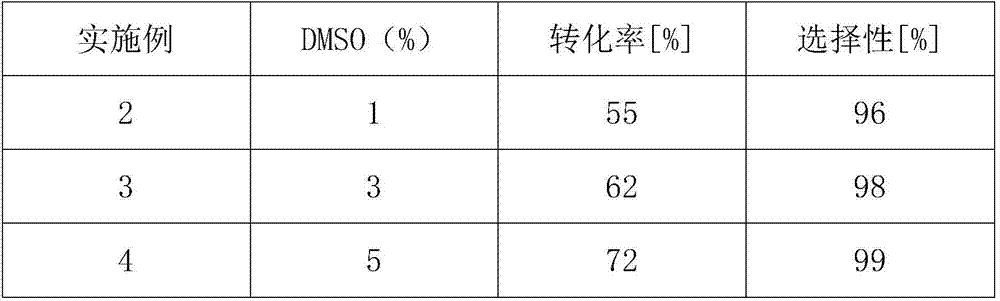
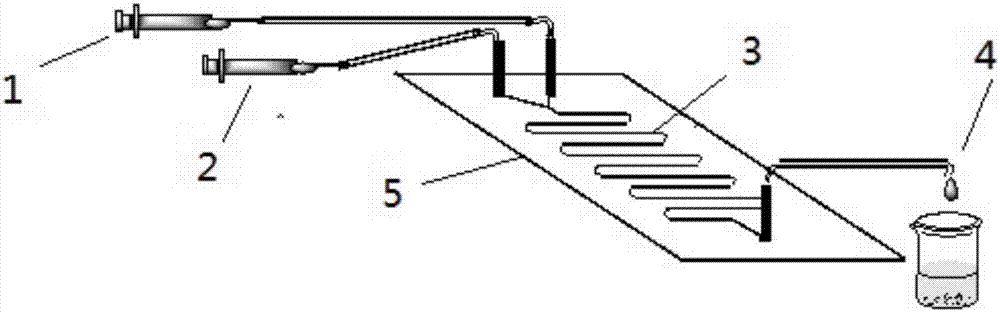
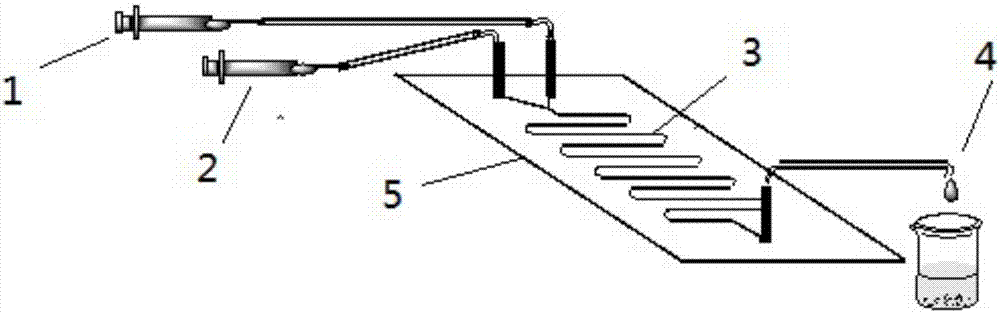
The invention discloses a method for online synthesizing 5'-O-palmitoyl uridine in a lipozyme catalysis mode .The method includes the steps that dimethyl sulfoxide and tert-amyl alcohol with the volume ratio of 1:(8-16) serve as a reaction solvent, uridine and palmitic acid vinyl ester with the molar ratio of 1:(5-13) serve as raw materials, 0.5 g to 1.0 g of lipozyme TLIM serves as a catalyst, the raw materials and the reaction solvent are placed into an injector, a reaction channel of a microfluidics channel reactor is evenly filled with the lipozyme TLIM, and the raw materials and the reaction solvents are continuously led into a reaction channel device under pushing of an injection pump for an acylation reaction, wherein the inner diameter of the reaction channel of the microfluidics channel reactor is 0.8 mm to 2.4 mm, the length of the reaction channel is 0.5 m to 1.0 m, the temperature of the acylation reaction is controlled to be 15 DEG C to 50 DEG C, the concentration of the uridine in the reaction system is 0.03 mmol / mL to 0.07 mmol / mL, and the time of the acylation reaction is 20 min to 35 min; reacted liquid is online collected through a product collector and subjected to conventional aftertreatment, and the 5'-O-palmitoyl uridine is obtained .The method has the advantages of being short in reaction time and high in selectivity and yield.
Owner:ZHEJIANG FORESTRY UNIVERSITY
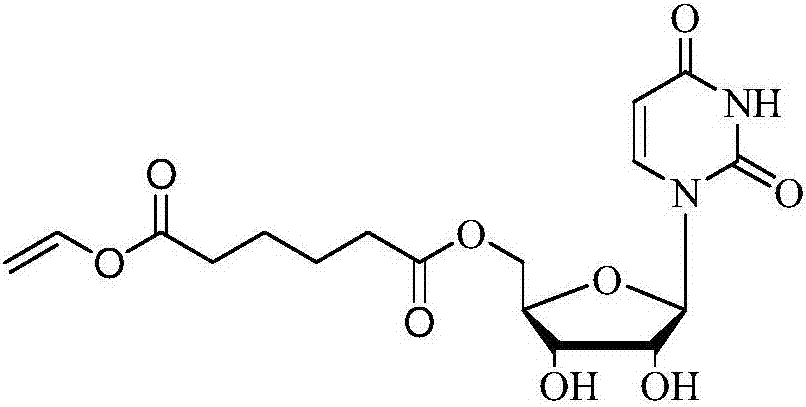
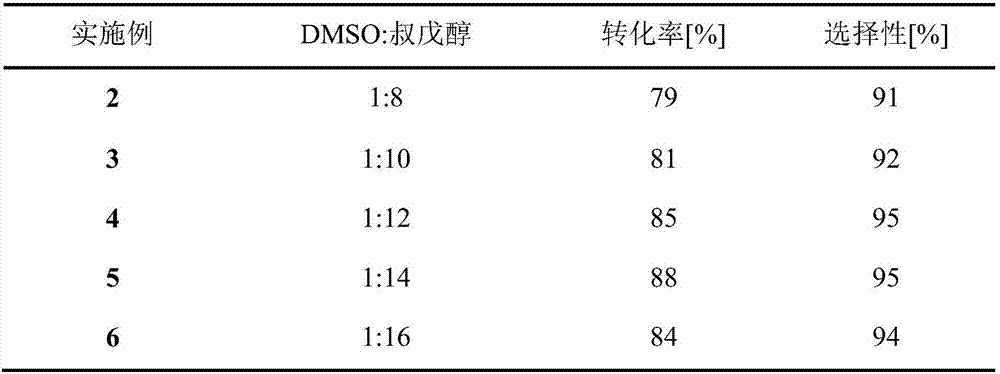
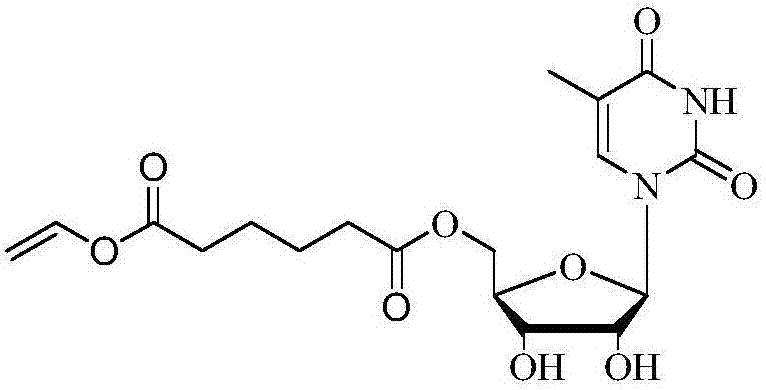
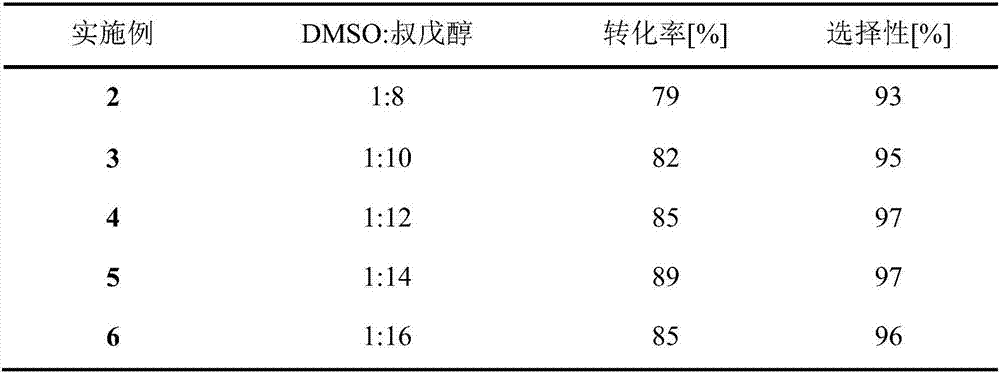
Method for 5'-O-ethylene adipyl uridine online synthesis through lipase catalysis
ActiveCN107384991AShort reaction timeImprove conversion rateBioreactor/fermenter combinationsBiological substance pretreatmentsReaction temperatureAdipic acid
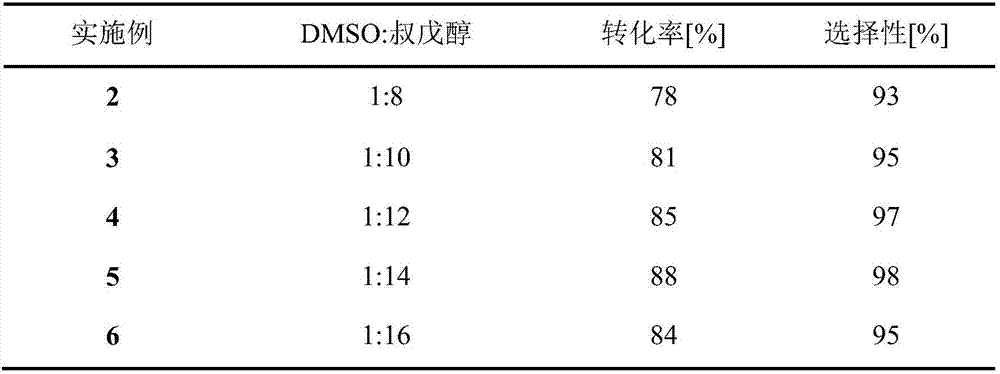
The invention discloses a method for 5'-O-ethylene adipyl uridine online synthesis through lipase catalysis. The method comprises the steps that dimethyl sulfoxide and tert-amyl alcohol serve as reactive solvents, uridine and adipic acid divinyl ester serve as raw materials, and 0.5-1.0 g of a lipase, namely Lipozyme TLIM, serves as a catalyst, wherein the volume ratio of the dimethyl sulfoxide to the tert-amyl alcohol is 1:(8-16), and the molar ratio of the uridine to the adipic acid divinyl ester is 1:(5-13). The raw materials and the reactive solvents are placed into an injector, and a reaction passage of a microfluidic passage reactor is uniformly filled with the lipase, namely the Lipozyme TLIM. Under pushing of an injection pump, the raw materials and the reactive solvents are continuously pumped into the reaction passage for acylation reaction. The inner diameter of the reaction passage of the microfluidic passage reactor is 0.8-2.4 mm, the length of the reaction passage is 0.5-1.0 m, the acylation reaction temperature is controlled to be 15-50 DEG C, and the acylation reaction time is 20-35 min. Through a product collector, reactive solution online collection is conducted, and after a reactive solution is subjected to conventional after-treatment, 5'-O-ethylene adipyl uridine is obtained. The method for 5'-O-ethylene adipyl uridine online synthesis through lipase catalysis has the advantages that the reaction time is short, the selectivity is high, and the productive rate is high.
Owner:ZHEJIANG UNIV OF TECH
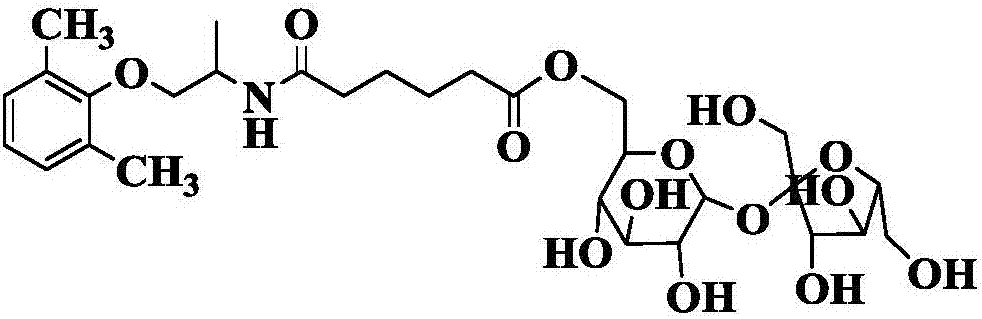
Method for synthesizing N-(5-sucrose ester valeryl)mexiletine online by lipozyme catalysis
InactiveCN107475329AShort reaction timeImprove conversion rateFermentationSucroseReaction temperature
The invention discloses a method for synthesizing N-(5-sucrose ester valeryl)mexiletine online by lipozyme catalysis. The method takes N-(5-vinyl ester valeryl)mexiletine and sucrose as raw materials according to the amount of substance ratio of 1 to (1 to 10), dimethyl sulfoxide (DMSO) and tert-amyl alcohol as reaction solvents and lipozyme TL IM as a catalyst, and comprises the following steps: putting the raw materials and the reaction solvents into an injector; uniformly filling the lipozyme TL IM into a reaction channel of a micro-fluidic channel reactor; continuously introducing the raw materials and the reaction solvents into a reaction channel device under the pushing of the injector and carrying out esterification reaction, wherein the inner diameter of the reaction channel of the micro-fluidic channel reactor is 0.8mm to 2.4mm and the length of the reaction channel is 0.5m to 1.0m; controlling the reaction temperature to 20 DEG C to 60 DEG C and the reaction time to 20min to 40min; collecting a reaction solution online through a product collector and carrying out conventional post-treatment on the reaction solution to obtain the N-(5-sucrose ester valeryl)mexiletine. The method disclosed by the invention has the advantages of short reaction time, high selectivity and high yield.
Owner:ZHEJIANG UNIV OF TECH
Lipozyme-catalyzed on-line synthesizing method for 5'-O-ethylene hexanedioyl-5-methyluridine
InactiveCN107384781AShort reaction timeImprove conversion rateEnzyme production/based bioreactorsFermentationGramAdipic acid
The invention discloses a lipozyme-catalyzed on-line synthesizing method for 5'-O-ethylene hexanedioyl-5-methyluridine. The lipozyme-catalyzed on-line synthesizing method comprises the following steps that dimethyl sulfoxide and tert-amyl alcohol with the volume ratio being 1:8-16 are taken as a reaction solvent, 5-methyluridine and adipic acid divinyl ester with the molar ration being 1:5-13 are taken as raw materials, 0.5-1.0 gram of lipozyme TLIM is taken as a catalyst, the raw materials and the reaction solvent are placed in a syringe, a reaction channel of a microfluidic channel reactor is evenly filled with the lipozyme TLIM, the raw materials and the reaction solvent are continuously injected into the reaction channel for acylation reaction under driving of an injection pump, the inner diameter of the reaction channel of the microfluidic channel reactor is 0.8-2.4 mm, the length of the reaction channel is 0.5-1.0 m; and the temperature of the acylation reaction is controlled to be at 15-50 DEG C, the time for the acylation reaction is 20-35 minutes, a reaction solution is collected on line by a product collector, and the 5'-O-ethylene hexanedioyl-5-methyluridine is obtained after conventional treatment is conducted on the reaction solution. The lipozyme-catalyzed on-line synthesizing method has the advantages of being short in reaction time, high in selectivity and high in yield.
Owner:ZHEJIANG UNIV OF TECH
Method for synthesizing 5'-O-ethylene hexanedioyl-5-floxuridine on line through catalyzing of lipase
InactiveCN107384782AShort reaction timeImprove conversion rateEnzyme production/based bioreactorsFermentationAdipic acidSolvent
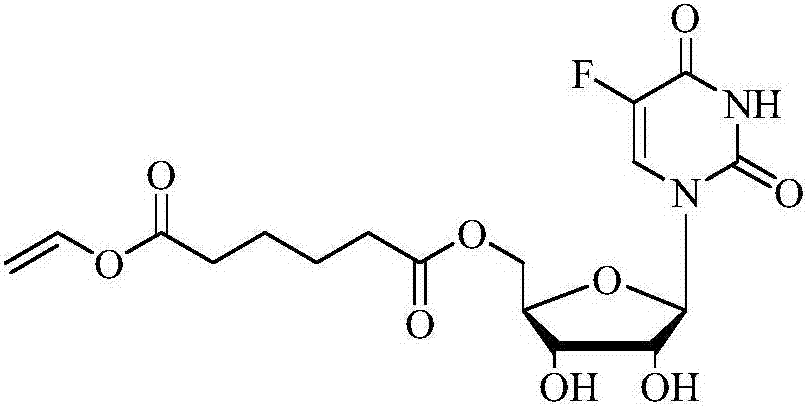
The invention discloses a method for synthesizing 5'-O-ethylene hexanedioyl-5-floxuridine on line through catalyzing of lipase. The method comprises the steps that dimethylsulfoxide and tert-amyl alcohol are used as reactive solvents, wherein the volume ratio of the dimethylsulfoxide to the tert-amyl alcohol is 1:(8-16); 5-floxuridine and adipic acid divinyl ester are used as raw materials, wherein the mole ratio of the 5-floxuridine to the adipic acid divinyl ester is 1:(5-13); 0.5-1.0 g of the lipase Lipozyme TLIM is used as a catalyst; the raw materials and the reactive solvents are put into an injector, a reaction channel of a microfluidic channel reactor is uniformly filled with the lipase Lipozyme TLIM, and under pushing of an injection pump, the raw materials and the reaction solvents are continuously injected into the reaction channel to conduct an acylation reaction, wherein the inner diameter of the reaction channel of the microfluidic channel reactor is 0.8-2.4 mm, and the length of the reaction channel is 0.5-1.0 m; the temperature of the acylation reaction is controlled to be 15-50 DEG C, and the time of the acylation reaction is 20-35 min; and reaction liquid is collected on line through a product collector and conventionally post-processed, and thus the 5'-O-ethylene hexanedioyl-5-floxuridine is obtained. The method has the advantages of short reaction time, high selectivity and high yield.
Owner:ZHEJIANG UNIV OF TECH
Method for synthesizing alpha-alkyl ketone under catalysis of iridium
InactiveCN106478395AMeet the requirements of green chemistryOrganic compound preparationCarbonyl compound preparationIridiumEvaporation
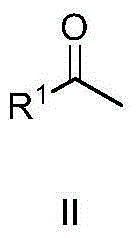
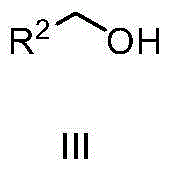
The invention discloses a method for synthesizing alpha-alkyl ketone under catalysis of iridium. The method comprises steps as follows: ketone, an alcohol compound, a iridium complex catalyst, alkali and a solvent, namely, tert-amyl alcohol are added to a reaction container, the reaction mixture is subjected to a reflux reaction in the air and cooled to the room temperature after the reaction ends, a solvent is removed through rotary evaporation, and a target compound is obtained through column separation. A tridentate iridium complex with an N^C^N ligand is used, all that is required is to add 0.2 equivalents of carbonate during the reaction in the air, the reaction takes only 10-12 h, and remarkable advantages are shown. Therefore, the reaction meets the green chemistry requirement, and broad development prospect is realized.
Owner:NANJING UNIV OF SCI & TECH
Method for synthesizing N,N'-dialkylurea
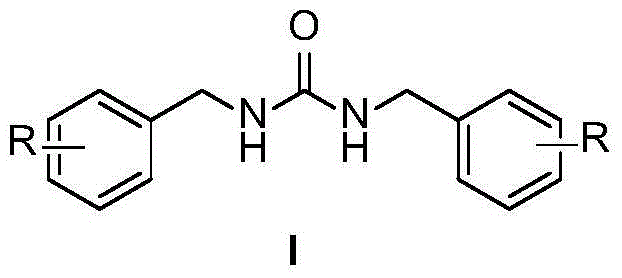
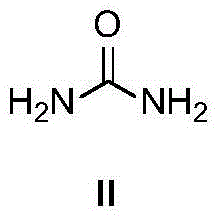
InactiveCN104557618AMeet the requirements of green chemistryUrea derivatives preparationOrganic compound preparationIridiumRoom temperature
The invention discloses a method for synthesizing N,N'-dialkylurea. The method comprises the following steps: adding urea, benzyl alcohol, a transition metal catalyst iridium complex, alkali and tert-amyl alcohol in a reaction container, performing reaction on a reaction mixture in a microwave reactor for several hours at 100-130 DEG C, and then cooling to room temperature. Compared with the prior art, the method disclosed by the invention is started from the raw material, namely urea, which is simple and easy to obtain, N,N'-dialkylurea is obtained by performing coupling reaction with alcohol which is more environment-friendly; furthermore, a generated byproduct is water, so that the reaction is in line with the requirements of green chemistry and has broad development prospects.
Owner:NANJING UNIV OF SCI & TECH
Double metal complex catalyst and its prepn
InactiveCN1486788AHigh activityReduce dosageOrganic-compounds/hydrides/coordination-complexes catalysts(Hydroxyethyl)methacrylatePolyol
The double metal complex catalyst (DMC) consists of Zn3[Co(CN)6]2íñaZnC12íñbH2Oíñc organic small molecular double-alcohol ligand and macro molecular nitric propyl dine ligand. The double alcohol is one of tert-butyl alcohol-tert-propyl alcohol, tert-butyl alcohol-tert-amyl alcohol, tert-butyl alcoho-n-butyl alcohol, tert-butyl alcohol-benzyl alcohol, etc. The double metal complex catalyst product has high activity, high stability, low consumption and long storage life over two years. The double metal complex catalyst is used in synthesizing poly(ether polyol) with great molecular weight, low unsaturation degree and relatively narrow molecular weight distribution, and it is one ideal material for preparing high performance polyurethane.
Owner:LIMING RES INST OF CHEM IND
Method for preparation of tert-amyl alcohol by isoamylene hydration
InactiveCN108017508ARaise the ratioReduce the ratioPreparation by hydroxy group additionHydration reactionFixed bed
The invention discloses a method for preparation of tert-amyl alcohol by isoamylene hydration. The method specifically includes the steps of: mixing an isoamylene component rich in 2-methyl-1-butene and 2-methyl-2-butene, water and acetone, then loading the mixture into a fixed bed reactor holding a strong acidic cation exchange resin catalyst, and carrying out hydration reaction to obtain tert-amyl alcohol. The method provided by the invention uses acetone as the solvent, which is free of azeotropy with water and is easy to recover; the solvent ratio is low, the energy consumption of reactionproduct post-treatment is saved, and the production operation cost is also saved.
Owner:CHINA PETROLEUM & CHEM CORP +1
Method for preparing tert-amyl alcohol
InactiveCN107879894AImprove the mixing effectIncrease mass transfer rateOrganic compound preparationOrganic-compounds/hydrides/coordination-complexes catalystsHydration reactionReaction rate
The invention discloses a method for preparing tert-amyl alcohol. The method disclosed by the invention comprises the following steps: enabling isopentene-enriched materials, water and promoter mixtures to simultaneously enter a static mixer, mixing, and introducing the mixed materials to enter a fixed bed reactor filled with a strong acid cation exchange resin to carry out hydration reaction; andsimply distilling and rectifying after the reaction is ended, thereby obtaining the high-purity product (tert-amyl alcohol) on a column reactor. According to the method disclosed by the invention, tetrabutylammonium bromide is added into a hydrate system to serve as the promoter, the contact environment between isopentene and an active center on the catalyst surface is improved, the liquid film lipophilicity on the catalyst surface is improved, contact resistance between isopentene and the catalyst surface is reduced, diffusion of isopentene to the active center of the catalyst surface is facilitated, and the reaction rate is further improved.
Owner:CHINA PETROLEUM & CHEM CORP +1
Extractant with high distribution coefficient for polyhydric phenol in phenolic wastewater and extraction method
ActiveCN107585820AWide ratio rangeEasy to removeWater contaminantsWater/sewage treatment by extractionRaffinatePhenol
The invention belongs to the technical field of sewage treatment and discloses an extractant with high distribution coefficient for polyhydric phenol in phenolic wastewater and an extraction method. The method comprises the following steps: using 30-99% by volume of methyl n-propyl ketone as a main extractant and 1-70% by volume of tert-amyl alcohol as a synergistic extractant to obtain a composite extractant; and carrying out multi-stage counter-current extraction on phenolic wastewater by the use of the composite extractant so as to obtain an extract phase and a raffinate. Removal efficiencyof polyhydric phenol in phenolic wastewater by the composite extractant is greatly raised, and the composite extractant has low boiling point and low recycling energy consumption. The composite extractant has wide range of volume ratio, is simple for industrial operation, and has a good prospect in actual industrial application.
Owner:SOUTH CHINA UNIV OF TECH
Method for synthesizing alpha-alkyl ketone
InactiveCN105439787AMeet the requirements of green chemistryOrganic compound preparationCarbonyl compound preparationIridiumBifunctional
The invention discloses a method for synthesizing alpha-alkyl ketone. The method comprises the following steps: adding ketone, a compound alcohol, an iridium complex catalyst, an alkali and a solvent tert-amyl alcohol in a reaction container, carrying out a refluxing reaction on the above obtained reaction mixture in air for several hours, cooling the obtained reaction product to room temperature, carrying out rotary evaporation to remove the solvent, and carrying out column separation to obtain the target compound. The meta-organic bifunctional iridium complex is used, only 0.1 equivalent carbonate is added in the reaction process, and the reaction is carried in air for 6h, so obvious advantages are displayed; and the reaction accords with green chemistry requirements, and has wide development prospect.
Owner:NANJING UNIV OF SCI & TECH
Method of using lipase to catalyze and synthesize mannose-6-palmitate on line
ActiveCN103184254AReduce usageShort reaction timeBioreactor/fermenter combinationsBiological substance pretreatmentsPalmitatesSolvent
The invention discloses a method of using lipase to catalyze and synthesize mannose-6-palmitate on line. The method comprises the following steps: taking mannose and vinyl palmitate as raw materials according to molar ratio of 1:3-8, taking 0.5-1.0 g of lipase Lipozyme TLIM as a catalyst, taking tert-amyl alcohol and DMSO (dimethyl sulfoxide) mixed solvent as a reaction solvent, uniformly filling the lipase Lipozyme TLIM into a reaction channel of a microfluidic channel reactor, wherein the inner diameter of the reaction channel of the microfluidic channel reactor is 0.8-2.4 mm, and the reaction channel is 0.5-1.0 m long, continuously introducing the raw materials and the reaction solvent into the reaction channel to perform acylation reaction, controlling the temperature of acylation reaction to be 40-55 DEG C, and keeping the acylation reaction for 15-35 min, on line collecting reaction liquid, and conventionally post-processing the reaction liquid to obtain mannose-6-palmitate. The method has the advantages of short reaction time, high selectivity and high yield.
Owner:ZHEJIANG UNIV OF TECH
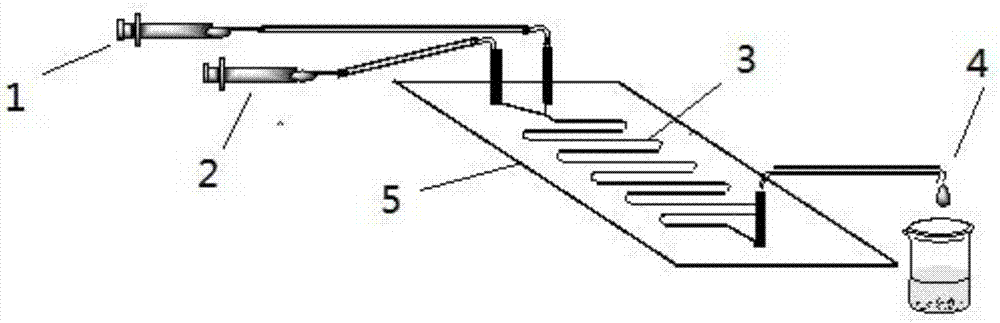
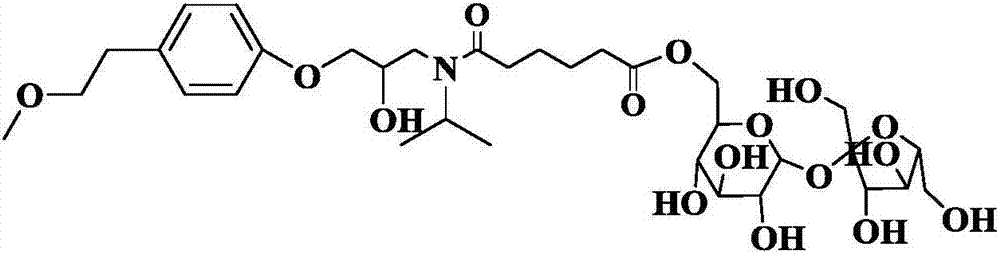
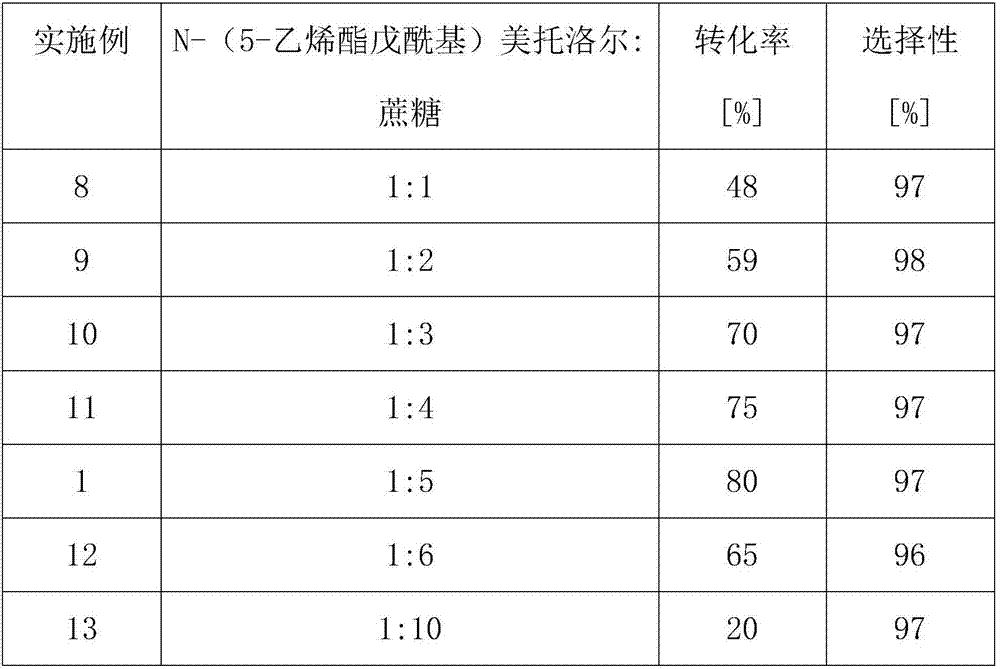
Method for online synthesis of N-(5-sucrose ester valeryl)metoprolol by means of catalysis of lipase
The invention discloses a method for online synthesis of N-(5-sucrose ester valeryl)metoprolol by means of catalysis of lipase. The method comprises the following steps: putting N-(5-vinyl ester valeryl)metoprolol and sucrose, which is taken as raw materials, as well as dimethyl sulfoxide (DMSO) and tert-amyl alcohol, taken as reaction solvents, into an injector, wherein the ratio of amount of substance of the N-(5-vinyl ester valeryl)metoprolol to the sucrose is equal to 1 to (1-10); evenly filling a reaction channel of a microfluidic channel reactor with the lipase Lipozyme TL IM taken as a catalyst; continuously injecting the raw materials and the reaction solvents into the reaction channel under the propelling of an injection pump, and carrying out an esterification reaction, wherein the inner diameter of the reaction channel of the microfluidic channel reactor is 0.8-2.4mm, and the length of the reaction channel is 0.5-1.0m; controlling the temperature of the esterification reaction to be 20-60 DEG C and the time of the esterification reaction to be 20-40min, collecting reaction liquid in an online way by using a product collector, and carrying conventional aftertreatment on the reaction liquid to obtain the N-(5-sucrose ester valeryl)metoprolol. The method provided by the invention has the advantages of being short in reaction time, high in selectivity and high in yield.
Owner:ZHEJIANG UNIV OF TECH
Method for synthesizing amide
InactiveCN107235852AMeet the requirements of green chemistryOrganic compound preparationCarboxylic acid amides preparationEvaporationDiol
The invention discloses a method for synthesizing amide. The method includes the steps: adding dihydric alcohol, Iridium complex catalysts and solvent tert-amyl alcohol into a reaction vessel; performing reflux reaction on reaction mixture in oil bath, and adding compound amine several hours after; continuing to react for several hours, and cooling reaction mixture to reach indoor temperature; performing rotary evaporation to remove solvents, and performing column separation to obtain a target compound. The method adopts bi-functional catalysts of ligands with dipyridyl, the dihydric alcohol serves as a raw material, the catalysts and the dihydric alcohol directly react in air for 18 hours to obtain the target compound, alkaline environments are omitted, obvious advantages are shown, requirements of green chemistry are met, and the method has a wide development prospect.
Owner:NANJING UNIV OF SCI & TECH
Manufacturing method of granular sodium tert-pentoxide
ActiveCN102001915AAvoid harmReduce security risksPreparation of metal alcoholatesOrganic solventNitrogen
The invention discloses a manufacturing method of granular sodium tert-pentoxide, which comprises the following steps: taking metal sodium, tert-amyl alcohol and inert organic solvent as raw materials, pouring the tert-amyl alcohol and the inert solvent into a reaction kettle, further pouring the metal sodium into the reaction kettle, carrying out reflux reaction for 8-40h under the protection of nitrogen, evaporating the tert-amyl alcohol under normal pressure, decompressing to minus 0.08-minus 0.098MPa after the tert-amyl alcohol is completely evaporated, evaporating the residual inert organic solvent, then regulating the stirring rate of the reaction kettle to 10-80 turns / minute, decompressing and evaporating the inert organic solvent, cooling a remaining granular sodium tert-pentoxide product in the kettle to below 40 DEG C, and finally obtaining the required granular sodium tert-pentoxide. The manufacturing method can carry out production by utilizing general chemical equipment, the operation conditions are mild, the production process is simple, the used raw materials are convenient and easy to obtain, the product quality is stable and reliable, and the shelf life of the product can be effectively prolonged.
Owner:GENCHEM & GENPHARM CHANGZHOU CO LTD
Preparation method of mildew-proof waterproof paint
InactiveCN108441009AImprove waterproof performanceImprove temperature resistanceAntifouling/underwater paintsPaints with biocidesPolyacrylamidePOLYOXYETHYLENE ETHER
The invention provides a preparation method of mildew-proof waterproof paint. The preparation method comprises the following steps: mixing butyl acrylate, vinyltriethoxysilane, tert-amyl alcohol and carboxylic butadiene-styrene latex, stirring and mixing at a rotation speed of 1000-2000 revolutions per minute for 40-60 minutes to obtain a mixed solution A for later use; mixing tetraethyl orthosilicate, nano-silver, nano-copper, nano-zinc oxide and citric acid, stirring and mixing at the rotation speed of 1000-2000 revolutions per minute for 30-70 minutes to obtain a mixed solution B for lateruse; mixing the mixed solution A and the mixed solution B, regulating the pH value to be 7.8-8.8, raising the temperature to 80-100 DEG C, adding nonylphenol polyoxyethylene ether and 1,2-benzisothiazole, and stirring at a rotation speed of 1500-2500 revolutions per minute for 25-35 minutes; adding polyacrylamide and hydroxyethylmethylcellulose, regulating the temperature to 60-80 DEG C, stirringat a rotation speed of 1000-2000 revolutions per minute for 30-50 minutes, and cooling, thereby obtaining the mildew-proof waterproof paint.
Owner:SUZHOU JUKANG NEW MATERIAL TECH
Tert-amyl alcohol sodium preparing process and molten sodium dispersant
InactiveCN1986508AFast dissolutionImprove dissolution rateOrganic chemistryAluminium chlorideTitanium chloride
The present invention relates to tert-amyl alcohol sodium preparing process and molten sodium dispersant. During preparing tert-amyl alcohol sodium with sodium and tert-amyl alcohol as material, the metal salt with the general expression of MiAj is used as the dispersant, where, M represents 1-4 valent metal ion, A is acid radical, i is integral of 1-4 and j is integral of 1-3. The molten sodium dispersant of the present invention is calcium chloride, sodium chloride, titanium chloride, aluminum chloride, etc. and has sodium adsorbed onto its surface to increase the reaction surface area of sodium and speed the reaction of sodium and tert-amyl alcohol, with the reaction period being shortened to less than 12 hr.
Owner:DONGHUA UNIV
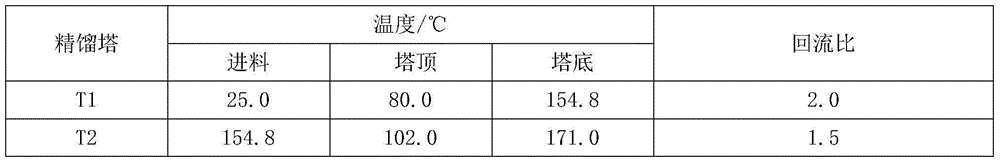
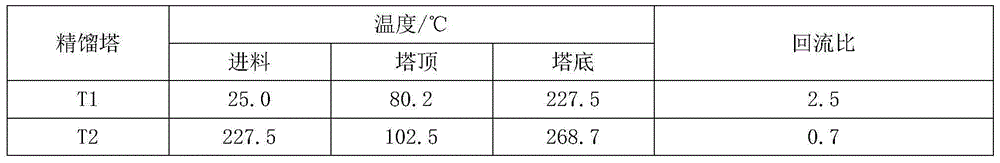
Method of separating mixture of tert-amyl alcohol and benzene by extractive distillation
ActiveCN105198701AReduce energy consumptionSimple processOrganic compound preparationDistillation purification/separationBenzeneExtractive distillation
The invention discloses a method of separating a mixture of tert-amyl alcohol and benzene by extractive distillation. According to the method, phenyl-ethanolamine compounds are taken as extraction agents, and the extraction agents are introduced to the upper part of an extractive distillation tower to fully contact with the mixture of tert-amyl alcohol and benzene introduced from the middle of the tower in the tower, then benzene is extracted from the top of the extractive distillation tower, and the extraction agents and tert-amyl alcohol are extracted from the bottom of the extractive distillation tower to enter an extraction agent recovery tower. Effective separation of the extraction agents and the tert-amyl alcohol is carried out in the extraction agent recovery tower, and the tert-amyl alcohol is extracted from the top of the extraction agent recovery tower, and the extraction agents are extracted from the bottom of the extraction agent recovery tower to be recycled. The method has the advantages of low energy consumption, simple process, realization of cyclic recovery of the extraction agents, high purity of products after the separation and the like.
Owner:QINGDAO UNIV OF SCI & TECH
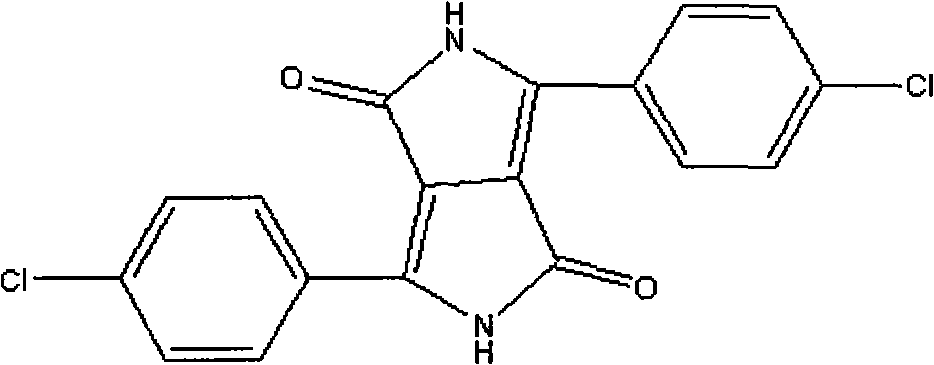
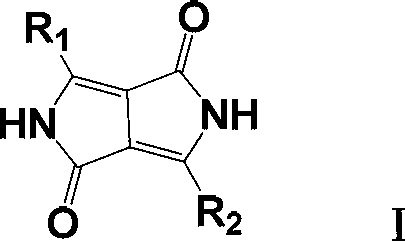
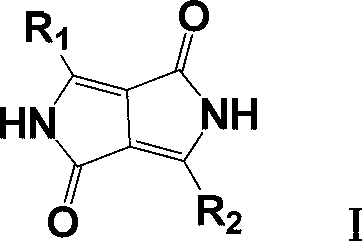
Improved method for preparing pyrrolopyrrole-1,4-diketone derivative
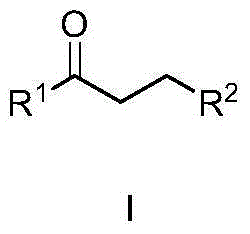
ActiveCN103012409ASimplify recycling stepsReduce manufacturing costOrganic chemistryAzo dyesPotassiumSuccinic acid
The invention relates to a method for preparing a pyrrolopyrrole-1,4-diketone derivative (the pyrrolopyrrole-1,4-diketone derivative can be used for preparing organic pigments). The method includes the main step: a target object is obtained through the reaction of succinic acid dialkyl ester and a nitrile compound at a temperature of 60 DEG C-140 DEG C in a sodium tert-pentoxide or potassium / tert-amyl alcohol medium. The method is characterized in that firstly, the adopted succinic acid dialkyl ester is succinic acid di-tert amyl ester; and secondly, after the reaction of succinic acid ditertiary amyl ester and the nitrile compound is stopped, the obtained mixture is heated and dried at the vacuum degree of 1-2 mmHg, the escaped tert-amyl alcohol is recovered at the same time, after heating and drying, the mixture heated and dried is transferred to a hydrolyzing reactor and diluted by carbinol / water, the generated sodium hydroxide is neutralized through inorganic acid, and the target object is obtained through liquid / solid separation. According to the invention, the steps for tert-amyl alcohol recovery are simplified, further, the cost in preparing the pyrrolopyrrole-1,4-diketone derivative is reduced.
Owner:辽宁鸿港化工有限公司 +2
Technology of synthesizing tert-amyl alcohol based on catalytic distillation solvent method
PendingCN110172013AImprove conversion rateHigh selectivityOrganic compound preparationChemical industryChemical synthesisHydration reaction
The invention belongs to the technical field of chemical synthesis and particularly relates to a technology of hydrating tert-amyl alcohol based on a C5 fraction catalytic distillation solvent method.The technology of synthesizing tert-amyl alcohol based on the catalytic distillation solvent method comprises the steps of performing hydration reaction in a catalytic distillation tower to form required tert-amyl alcohol by taking C5 fraction rich in tert-pentene and water as raw materials and strong-acid cation exchange resin as a catalyst in the presence of an amphiprotic solvent, namely an ethylene glycol butyl ether solvent. Compared with commonly used catalytical technologies using an alcohol solvent or not using a solvent, and the traditional fixed bed hydration technology, the technology of generating tert-amyl alcohol increases a conversion rate of tert-pentene to above 80%, much greater than 48% of a fixed bed reactor; the selectivity of the whole reaction is further improved (from 90.3% to above 99%); the purpose of deep conversion of tert-pentene can be achieved; and at the same time, the whole technology has the advantages of simple flow and low energy consumption.
Owner:CHINA PETROLEUM & CHEM CORP +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com