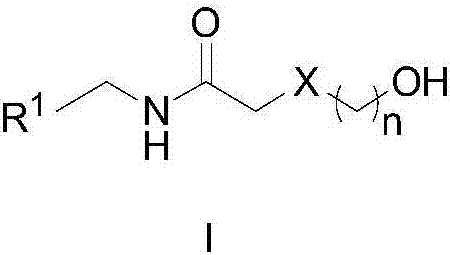
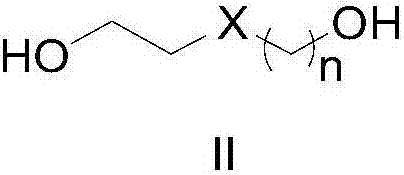
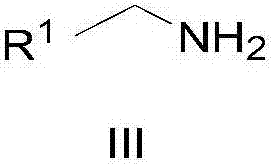
Method for synthesizing amide
A synthetic method and amide technology, applied in the formation/introduction of amide groups, carboxylic acid amide preparation, chemical instruments and methods, etc., to achieve the effect of broad development prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Example 1: N-benzyl-4-hydroxybutyramide
[0026] N‐benzyl‐4‐hydroxybutanamide
[0027]
[0028] 1,4-Butanediol (91 mg, 1 mmol), cat.[Ir] (5.4 mg, 0.01 mmol, 1 mol%) and tert-amyl alcohol (1 ml) were sequentially added into a 5 mL round bottom flask. After the reaction mixture was refluxed in air for 6 hours, benzylamine (118 mg, 1.1 mmol) was added, and the reaction was continued for 12 hours, then cooled to room temperature. Rotary evaporation removes solvent, then obtains pure target compound by column chromatography (developing solvent: sherwood oil / ethyl acetate), productive rate: 90%
[0029] 1 H NMR (500MHz, CDCl 3 )δ7.36‐7.27 (m, 5H), 5.94 (br, 1H), 4.44 (d, J = 5.7Hz, 2H), 3.71 (t, J = 5.8Hz, 2H), 2.39 (t, J = 6.6 Hz,2H),1.91(q,2H); 13 C NMR (125MHz, CDCl 3 )δ173.5, 138.0, 128.6, 127.6, 127.4, 61.9, 43.5, 33.6, 28.1.
Embodiment 2
[0030] Example 2: N-(4-methylbenzyl)-4-hydroxybutanamide
[0031] N‐(4‐methylbenzyl)‐4‐hydroxybutanamide
[0032]
[0033] 1,4-Butanediol (91 mg, 1 mmol), cat.[Ir] (5.4 mg, 0.01 mmol, 1 mol%) and tert-amyl alcohol (1 ml) were sequentially added into a 5 mL round bottom flask. After the reaction mixture was refluxed in air for 6 hours, 4-methylbenzylamine (133 mg, 1.1 mmol) was added, and the reaction was continued for 12 hours, then cooled to room temperature. Rotary evaporation removes solvent, then obtains pure target compound by column chromatography (developing solvent: sherwood oil / ethyl acetate), productive rate: 88%
[0034] 1 H NMR (500MHz, CDCl 3 )δ7.17‐7.13 (m, 4H), 5.97 (br, 1H), 4.39 (d, J = 5.7Hz, 2H), 3.69 (t, J = 5.8Hz, 2H), 2.37 (t, J = 6.7 Hz,2H),2.33(s,3H),1.89(q,2H); 13 C NMR (125MHz, CDCl 3 )δ173.5, 137.0, 135.0, 129.2, 127.6, 61.8, 43.2, 33.5, 28.1, 20.9.
Embodiment 3
[0035] Example 3: N-(3-methylbenzyl)-4-hydroxybutanamide
[0036] N‐(3‐methylbenzyl)‐4‐hydroxybutanamide
[0037]
[0038] 1,4-Butanediol (91 mg, 1 mmol), cat.[Ir] (5.4 mg, 0.01 mmol, 1 mol%) and tert-amyl alcohol (1 ml) were sequentially added into a 5 mL round bottom flask. After the reaction mixture was refluxed in air for 6 hours, 3-methylbenzylamine (133 mg, 1.1 mmol) was added, and the reaction was continued for 12 hours, then cooled to room temperature. Rotary evaporation removes solvent, then obtains pure target compound by column chromatography (developing solvent: sherwood oil / ethyl acetate), productive rate: 82%
[0039] 1 H NMR (500MHz, CDCl 3 )δ7.22‐7.19 (t, J=7.57Hz, 1H), 7.09‐7.04 (m, 3H), 6.15 (br, 1H), 4.37 (d, J=5.4Hz, 2H), 3.67 (t, J =5.9Hz, 2H), 2.37(t, J=6.6Hz, 2H), 2.33(s, 3H), 1.89(q, 2H); 13 C NMR (125MHz, CDCl 3 )δ173.3, 138.4, 137.9, 128.6, 128.5, 128.2, 124.7, 62.1, 43.6, 33.7, 28.1, 21.3.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com