Method for online synthesis of N-(5-sucrose ester valeryl)metoprolol by means of catalysis of lipase
A technology of ester valeryl and vinyl ester valeryl, applied in the field of lipase catalyzed on-line controllable selective synthesis of N-metoprolol, can solve the problems of low bioavailability, short half-life, long reaction time, etc. Effect of reaction time, reduced reaction cost, high conversion and selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
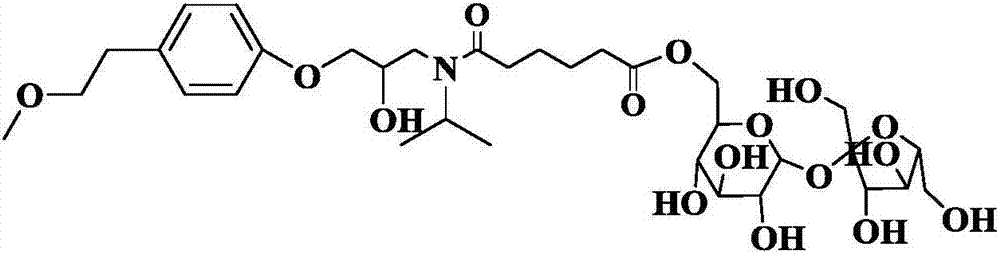
[0028] Embodiment 1: the synthesis of N-(5-sucrose ester pentanoyl) metoprolol
[0029]
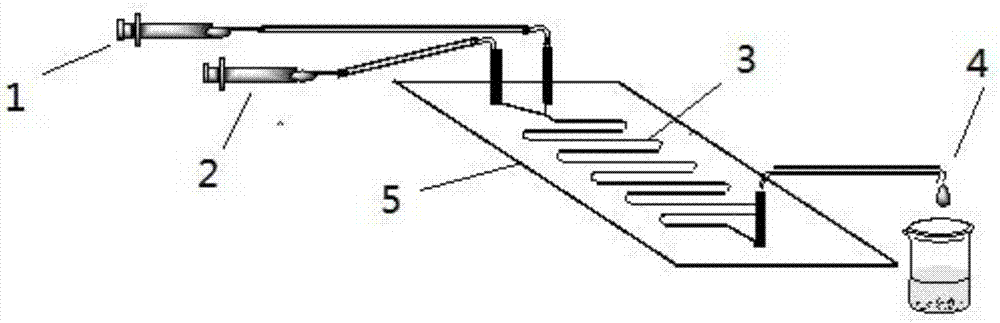
[0030] Dissolve N-(5-vinylvaleryl)metoprolol (0.1 mmol) in 0.70 mL DMSO and 9.30 mL tert-amyl alcohol, and dissolve sucrose (0.5 mmol) in 0.70 mL DMSO and 9.30 mL tert-amyl alcohol , and then packed in 10mL syringes for use. 0.87g of lipase Lipozyme TL IM was evenly filled in the reaction channel, driven by the PD 1200 syringe pump, the two reaction solutions were separated at 10.4 μL min -1 The flow rate enters the reaction channel through the "Y" joint for reaction, and the temperature of the reactor is controlled at 35 ° C by a water bath thermostat. The reaction solution flows continuously in the reaction channel for 30 minutes, and the reaction results are tracked and detected by thin-layer chromatography TLC.
[0031] Collect the reaction solution online by the product collector, remove the solvent by distillation under reduced pressure, use 200-300 mesh silica gel wet packing col...
Embodiment 2-7
[0036] Change the content of DMSO in the reaction medium (DMSO / tert-amyl alcohol) in the microfluidic channel reactor, control the temperature to 35°C, and the others are the same as in Example 1. The reaction results are shown in Table 1:
[0037] Table 1: The effect of the amount of DMSO in the reaction medium on the reaction
[0038] Example
[0039] The results in Table 1 show that when the flow rate is 10.4 μL min -1 , the reaction time is 30min, the reaction temperature is 35 ° C, the ratio of the amount of reactant N-(5-vinylvaleryl) metoprolol to sucrose is 1:5, and the conversion rate varies with the reaction system (DMSO / tert-amyl alcohol) in DMSO volume ratio increases and increases, when the amount of DMSO in DMSO and tert-amyl alcohol mixed solvent is 7%, reach optimum, then continue to increase the amount of DMSO in mixed solvent, will affect enzyme The activity affects the conversion and selectivity of the reaction. Therefore, the optimal reaction ...
Embodiment 8-13
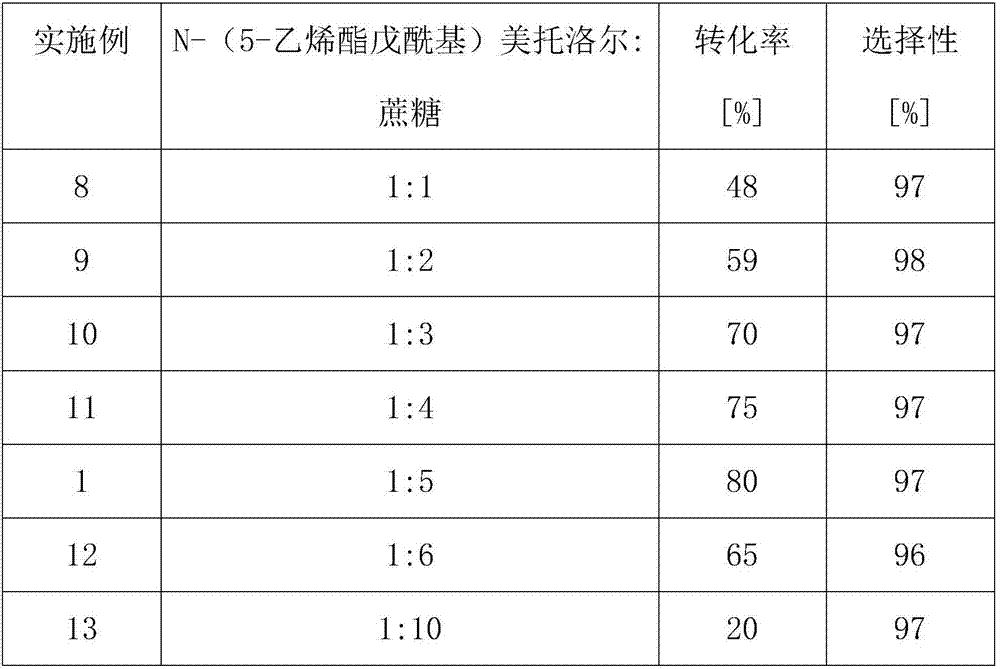
[0041] Change the ratio of the amount of N-(5-vinylvaleryl) metoprolol to the substrate substance of sucrose in the microfluidic microchannel reactor, control the temperature at 35° C., others are the same as in Example 1, and the results are shown in Table 2 Show:
[0042] Table 2: Effect of the ratio of the amount of N-(5-vinylvaleryl) metoprolol and sucrose substrate substance on the reaction
[0043]
[0044] The results in Table 2 show that when the flow rate is 10.4 μL min -1 , the reaction time is 30min, the reaction temperature is 35°C, and when the DMSO content of the reaction medium is 7%, with the increase of the amount of reactant sucrose, the conversion rate of the reaction also increases. When the ratio of the amount of the substrate substance is At 1:5, the conversion rate of the reaction is optimal, so the ratio of the amount of the optimal substrate substance in the microfluidic microchannel reactor in the present invention is 1:5.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com