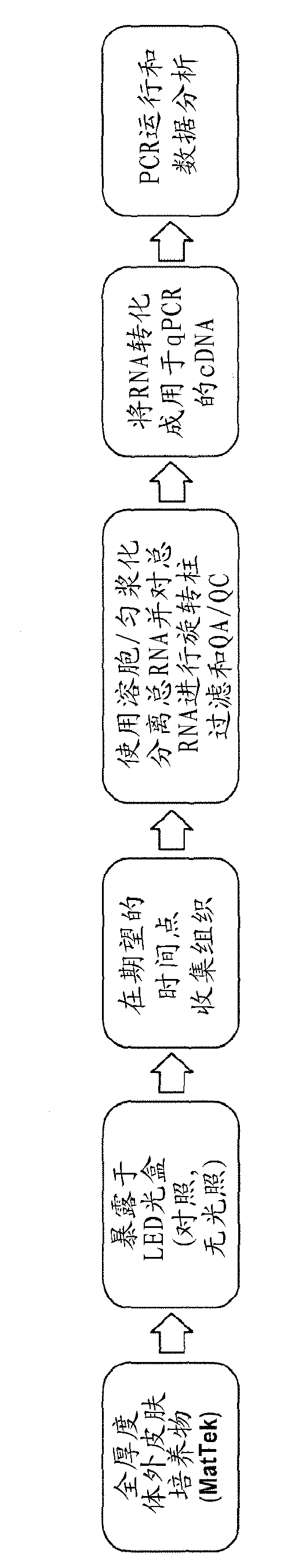
Compound, composition, and method for protecting skin from high energy visible light
A composition and compound technology, applied in the directions of skin care preparations, drug combinations, skin diseases, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] a) Synthesis:
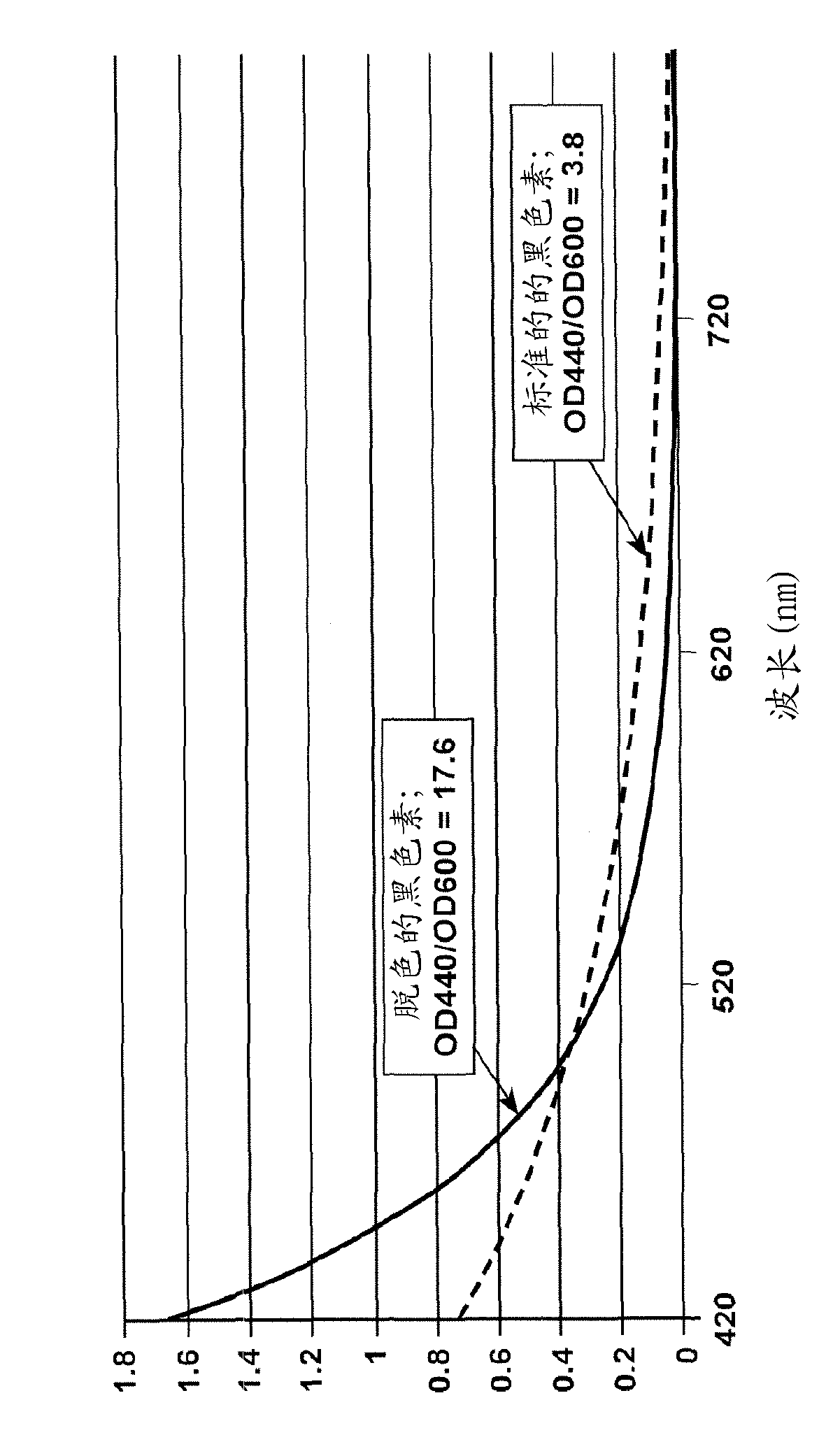
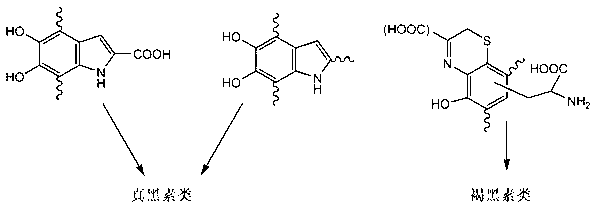
[0046] Melanin was synthesized as follows: 15g L-tyrosine was dissolved in 800mL water; 60g ammonium persulfate was dissolved in 200mL water; 26g sodium hydroxide (NaOH) was dissolved in 50mL water. The pH was adjusted to 8.5 with sodium hydroxide, and the solution was stirred for 10 hours.
[0047] b) Purification:
[0048] Under stirring, the product was acidified with hydrochloric acid (HCl) to pH 1.5 using about 1 L of water. Continue stirring for about 10 minutes, remove the stirrer, and let the product stand for 24 hours. The supernatant was separated, about 1 L of deionized water was added and the pH was adjusted to 2 with dilute hydrochloric acid, while stirring for 10 minutes; the product was allowed to stand for 24 hours without stirring.
[0049] c) Fractionation:
[0050] Raise the pH to between 3-4 with sodium hydroxide while stirring for about 1 hour, stop stirring and let the product stand for 24 hours. Separate the supernatant or collect the su...
example )
[0079] Additional UV filters (including combinations of any two or more of the following) are selected from the following categories (with specific examples): p-aminobenzoic acid, its salts and derivatives (ethyl ester, isobutyl ester) , Glyceryl ester; p-dimethylaminobenzoic acid); anthranilic acid (anthranilic acid esters; methyl ester, menthyl ester, phenyl ester, benzyl ester, phenethyl ester, agarwood Esters, terpinenyl esters and cyclohexenyl esters); salicylate esters (octyl ester, pentyl ester, phenyl ester, benzyl ester, menthyl ester (homosalate), glyceryl ester and Dipropanol ester); cinnamic acid derivatives (menthyl ester and benzyl ester, α-phenylcinnamonitrile; butyl cinnamoyl pyruvate); dihydroxycinnamic acid derivative (umbrella Ketone, methyl umbelliferone, methyl acetyl umbelliferone); camphor derivatives (3-benzylidene, 4-methylbenzylidene, polyacrylamidomethyl benzylidene ), benzalkonium methosulfate (benzalkonium methosulfate), benzalkonium camphor sulfon...
Embodiment 2
[0127] Example 2: Emulsion preparation
[0128]
[0129] 1) The order of premixing and adding the ingredients of the composition.
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com