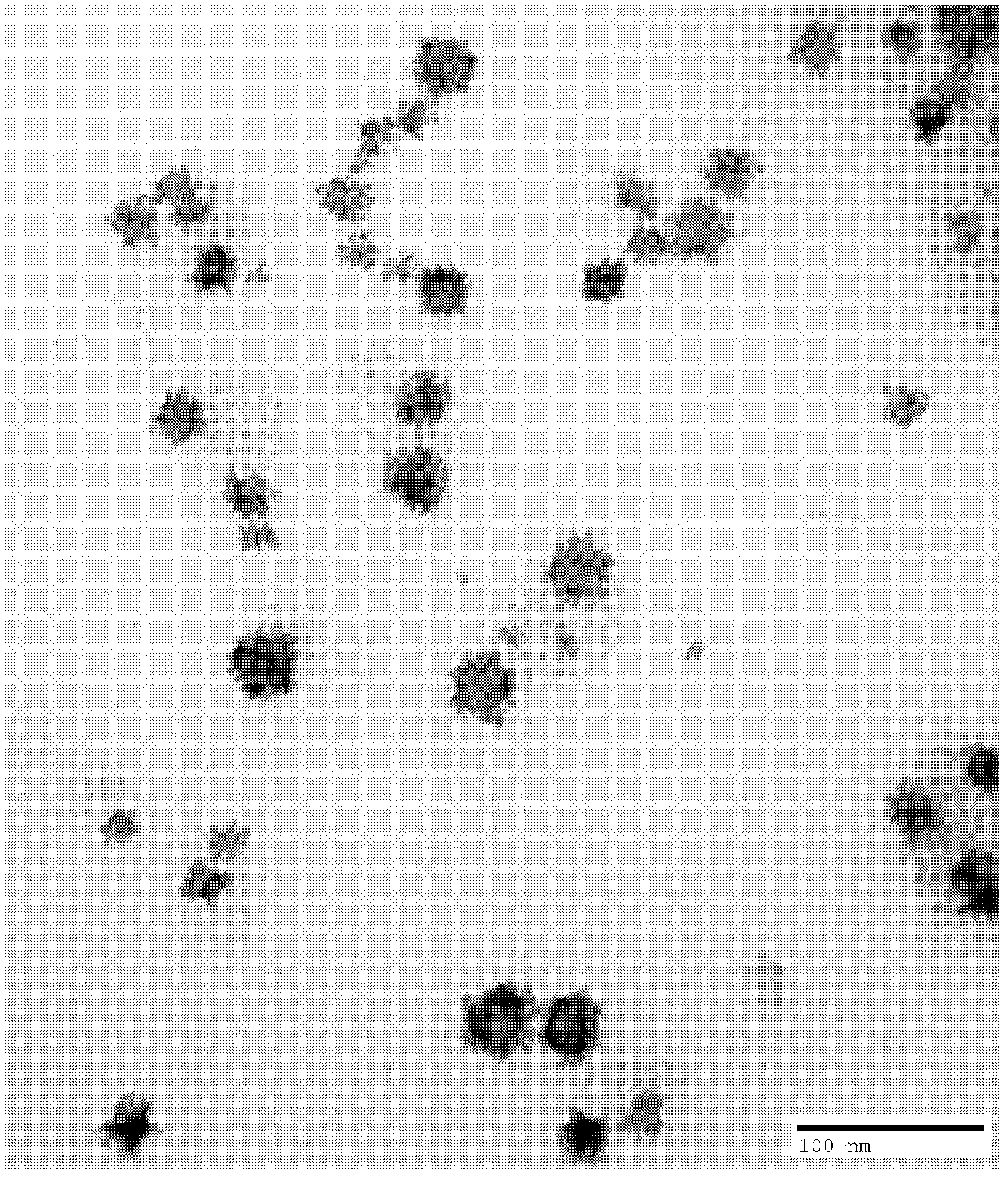Preparation method of Pd@Pt core-shell structural catalyst for low-temperature fuel cell
A technology of fuel cell and core-shell structure, which is applied in chemical instruments and methods, physical/chemical process catalysts, metal/metal oxide/metal hydroxide catalysts, etc. Large-scale production and other issues, to achieve the effect of simple preparation process, high electrochemical specific surface area, high oxygen reduction catalytic activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] 1. Add a certain amount of PdCl to a one-necked flask 2 aqueous solution and K 2 PtCl 4 Aqueous solution, so that the concentration of the sum of Pd and Pt elements is 15.1mmol L -1 , the atomic ratio of Pd and Pt is 1:2, stir evenly.
[0025] 2. Add to the above solution P123, stirring, the mass fraction of P123 after complete dissolution is 0.5%.
[0026] 3. Add ascorbic acid to the above solution so that the ratio of ascorbic acid to the sum of Pd and Pt elements is 4.8:1, and stir at room temperature for 12 hours.
[0027] 4. Centrifuge after the reaction, and then wash several times. The obtained metal particles were dispersed in isopropanol for later use.
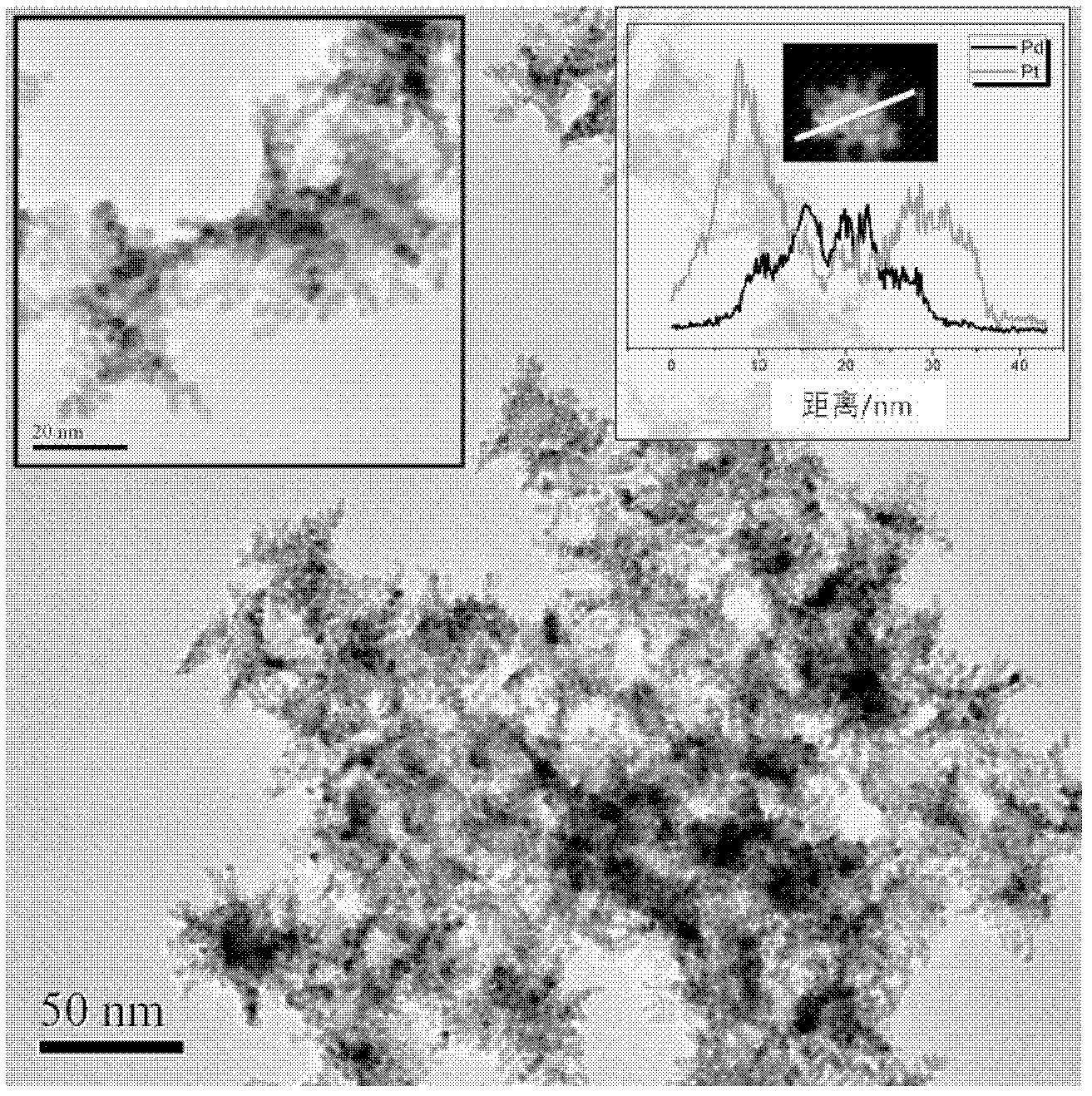
[0028] The TEM photo of the PdPt nanoparticles that the present embodiment obtains is as follows figure 1 shown.
Embodiment 2
[0030] 1. Add a certain amount of Na to the single-necked flask 2 PdCl 4 aqueous solution and H 2 PtCl 6 Aqueous solution, so that the concentration of the sum of Pd and Pt elements is 4.66mmol L -1 , the atomic ratio of Pd and Pt is 2:1, stir well.
[0031] 2. Add to the above solution F127, stir, the mass fraction of F127 after complete dissolution is 5%.
[0032] 3. Add ascorbic acid to the above solution so that the ratio of ascorbic acid to the sum of Pd and Pt elements is 15.6:1, and stir at room temperature for 12 hours.
[0033] 4. Centrifuge after the reaction, and then wash several times. The obtained metal particles were dispersed in isopropanol for later use.
[0034] The TEM photo of the PdPt nanoparticles that the present embodiment obtains is as follows figure 2 shown.
Embodiment 3
[0036] 1. Add a certain amount of Na to the single-necked flask 2 PdCl 4 aqueous solution and K 2 PtCl 4 Aqueous solution, so that the concentration of the sum of Pd and Pt elements is 4.66, 6.22, 7.77 and 9.33mmol L -1 , the corresponding atomic ratios of Pd and Pt are 2:1, 1:1, 2:3 and 1:2, and stir evenly.
[0037] 2. Add to the above solution F127, stir, the mass fraction of F127 after complete dissolution is 1%.
[0038] 3. Add ascorbic acid to the above solution so that the ratios of ascorbic acid to the sum of Pd and Pt elements are 15.6:1, 11.7:1, 9.4:1 and 7.8:1 respectively, and stir at room temperature for 12 hours.
[0039] 4. After the reaction, the reaction system was cooled to room temperature, then centrifuged and washed several times. The obtained metal particles were dispersed in isopropanol for later use.
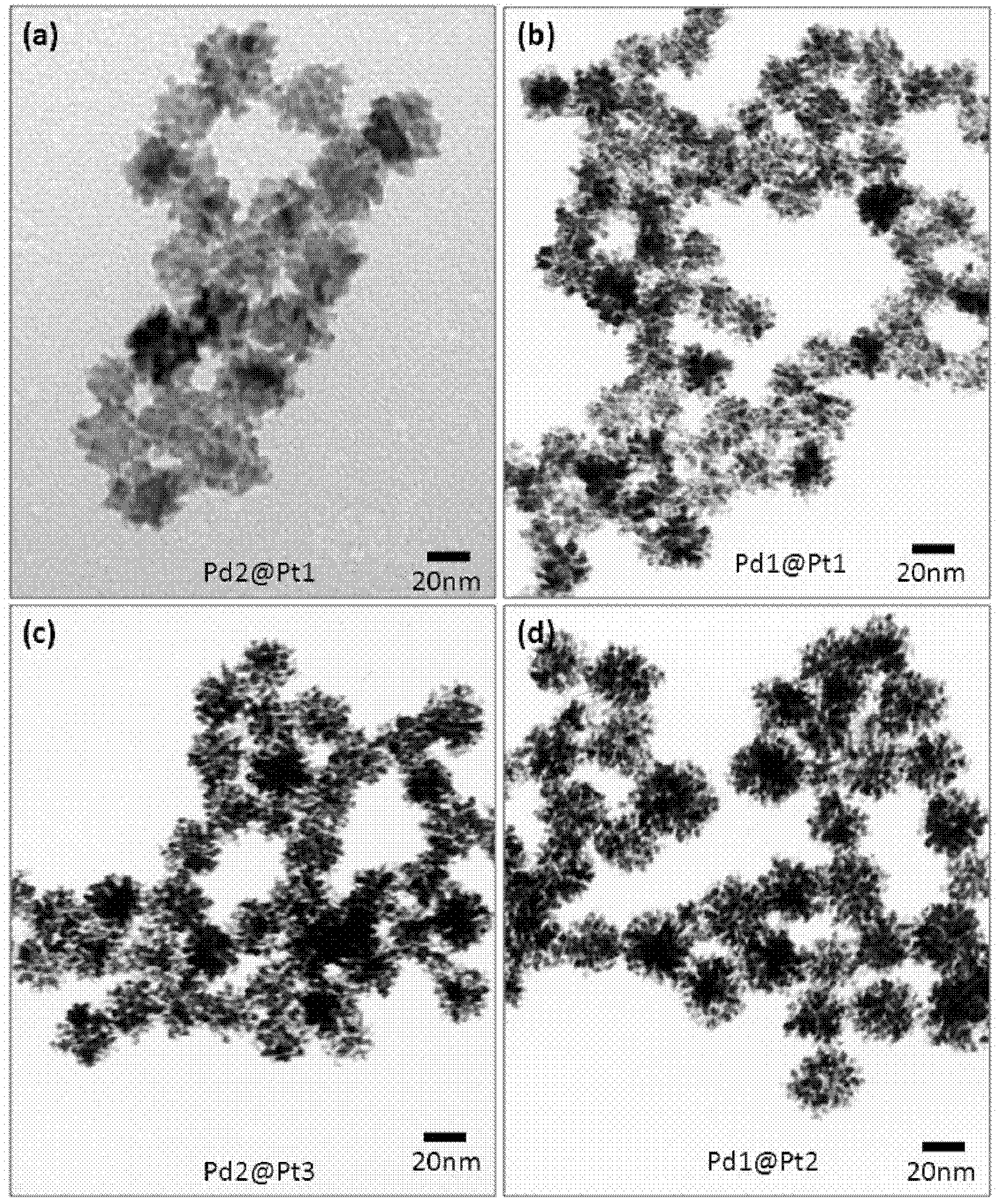
[0040] The TEM photo of the PdPt nanoparticles that the present embodiment obtains is as follows image 3 As shown, the atomic ratio obtained by...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com