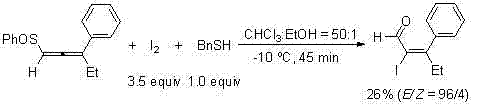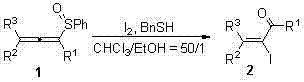High-selectivity synthesis method of alpha,beta-unsaturated iodoketenes and olefine aldehydes
A technology of iodoketene and high selectivity, which is applied in the high selectivity synthesis of α, to achieve the effects of high cis-trans selectivity, convenient preparation and mild conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Embodiment 1, synthesis ( Z )-4-iodo-5-nonanone-3-ene:
[0026]
[0027] Add 5-phenylsulfinyl-3,4-nonadiene (74.8 mg, 0.3 mmol), chloroform (1 mL), ethanol (30 µL) to the reaction tube. Place the reaction tube in a 40°C oil bath, add iodine (267.2 mg, 1.05 mmol), stir for 5 minutes, then add benzylthiol in chloroform (0.6 M, 0.5 mL). After stirring for 15 minutes, 6 mL of water was added to quench the reaction, and saturated sodium thiosulfate was added dropwise to neutralize excess iodine. Extract with ether (20 mL × 3), wash with saturated sodium chloride, and dry over anhydrous sodium sulfate. Filtration, concentration, flash column chromatography (eluent: petroleum ether ~ petroleum ether / ether = 300 / 1) to obtain the product ( Z )-4-iodo-5-nonanone-3-ene (66.1 mg, 82%): liquid.
[0028] 1 H NMR (300 MHz, CDCl 3 ) δ 6.99 (t, J = 6.9 Hz, 1 H, =CH), 2.83 (t, J = 7.4 Hz, 2 H, COCH 2 ), 2.49-2.37 (m, 2 H, CH 2 ), 1.69-1.56 (m, 2 H, CH 2 ), 1.44-1.23 (...
Embodiment 2
[0029] Embodiment 2, synthesis ( Z )-1-phenyl-3-iodo-4-octanone-2-ene:
[0030] According to the method described in Example 1, the difference is that the substrate used is: 1-phenyl-4-benzenesulfinyl-2,3-octadiene (93.1 mg, 0.3 mmol), 30 μL ethanol, 1 mL Chloroform, iodine (266.6 mg, 1.05 mmol), benzylthiol in chloroform (0.6 M, 0.5 mL) to give ( Z )-3-iodo-1-phenyl-4-octanone-2-ene (63.8 mg, 65%) (eluent: petroleum ether ~ petroleum ether / ether = 300 / 1 ~ petroleum ether / ether = 100 / 1): Liquid.
[0031] 1 H NMR (300 MHz, CDCl 3 ) δ 7.40-7.21 (m, 5 H, ArH), 7.11 (t, J = 6.9 Hz, 1 H, =CH), 3.77 (d, J = 6.6 Hz, 2 H, CH 2 Ar), 2.80 (t, J = 7.5 Hz, 2 H, COCH 2 ), 1.68-1.55 (m, 2 H, CH 2 ), 1.42-1.23 (m, 2 H, CH2 ), 0.90 (t, J = 7.5 Hz, 3 H, CH 3 ); 13 C NMR (75 MHz, CDCl 3 ) δ 194.9, 149.7, 137.1, 128.8, 128.6, 126.9, 113.1, 44.2, 37.5, 26.9, 22.2, 13.8; IR (neat) ν (cm -1 ) 3063, 3027, 2957, 2926, 2867, 1682, 1598, 1495, 1453, 1251, 1145, 1100; MS (...
Embodiment 3
[0032] Embodiment 3, synthesis ( Z )-2-methyl-4-iodo-5-nonanone-3-ene:
[0033]
[0034] According to the method described in Example 1, the difference is that the substrate used is: 1-methyl-5-benzenesulfinyl-3,4-nonadiene (79.1 mg, 0.3 mmol), 30 μL ethanol, 1 mL Chloroform, iodine (267.4 mg, 1.05 mmol), benzylthiol in chloroform (0.6 M, 0.5 mL) to give ( Z )-2-methyl-4-iodo-5-nonanone-3-ene (70.6 mg, 83%, 99.3% purity) (eluent: petroleum ether ~ petroleum ether / ether = 300 / 1): Liquid.
[0035] 1 H NMR (300 MHz, CDCl 3 ) δ 6.74 (d, J = 8.4 Hz, 1 H, =CH), 2.92-2.77 (m, 3 H, COCH 2 + CH), 1.77-1.56 (m, 2 H, CH 2 ), 1.44-1.23 (m, 2 H, CH 2 ), 1.12 (d, J = 6.6 Hz, 6 H, 2 × CH 3 ), 0.92 (t, J = 7.5 Hz, 3 H, CH 3 ); 13 C NMR (75 MHz, CDCl 3 ) δ 195.1, 157.1, 109.6, 37.5, 37.3, 27.0, 22.2, 20.7, 13.8; IR (neat) ν (cm -1 ) 2960, 2931, 2870, 1682, 1602, 1465, 1412, 1382, 1363, 1329, 1267, 1166, 1128, 1086; MS (70 eV, EI) m / z (%) 280 (M + , 0.76), 237 (100); H...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com