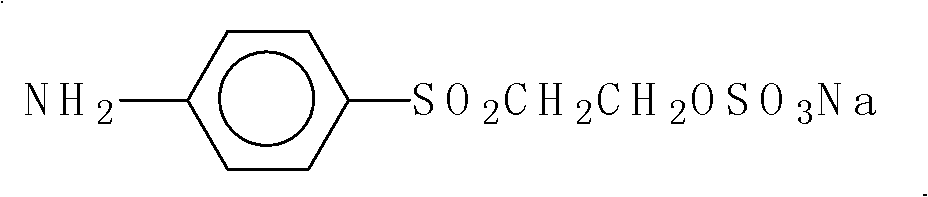Nylon reactive red dye
A reactive dye, nylon technology, applied in reactive dyes, azo dyes, organic dyes, etc., can solve the problem of unable to meet the needs of nylon dyeing, and achieve the effect of bright color, reducing pollution and reducing environmental pollution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
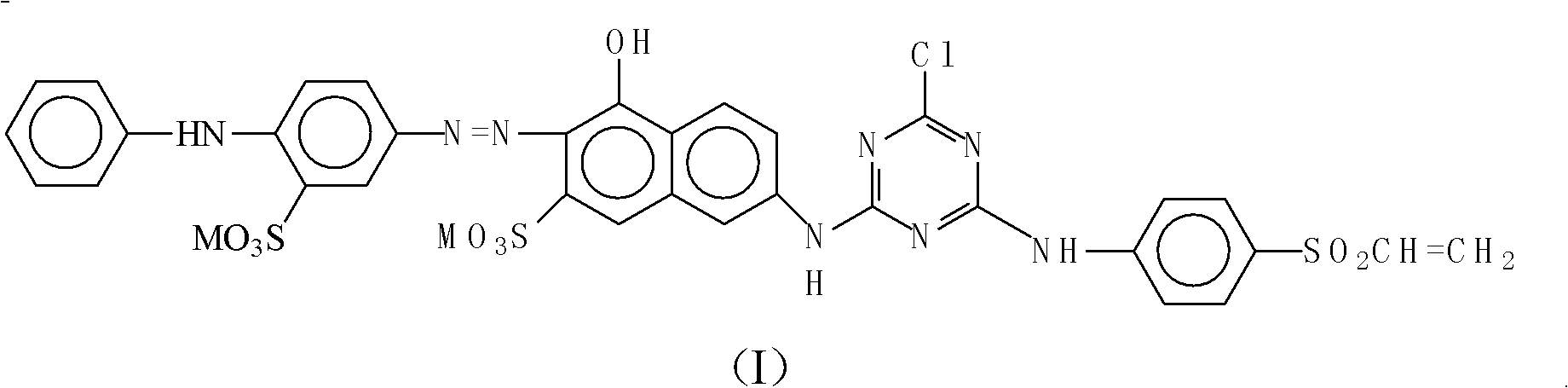
[0068] The preparation of the nylon active red dye compound of embodiment structural formula (II)
[0069]
[0070] a, the preparation of condensate:
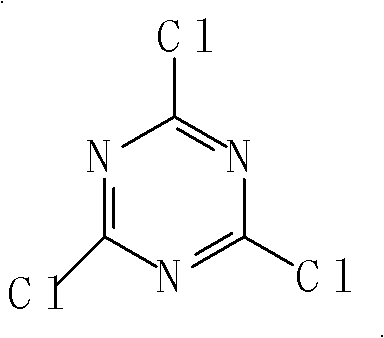
[0071] In the dissolution tank, add a small amount of bottom water and 30Kg of para-fat, and use NaHCO 3 Neutralize pH=4-5 to obtain para-ester solution; add a small amount of water and a small amount of crushed ice to the reaction kettle, then add 19Kg of cyanuric chloride, add para-ester dropwise while stirring, T=10-15°C , pH=2-3.5, the dropwise addition is completed, and react for 5 hours to obtain a condensation solution; add 26.5Kg of J acid to the condensation solution, adjust T=35-40°C, pH=5-6, and react for 3 hours to obtain Secondary condensate.
[0072] b, 4-aminodiphenylamine-2-sulfonic acid diazotization:
[0073] Add bottom water and 23.2Kg of 4-aminodiphenylamine-2-sulfonic acid to the reaction kettle, stir evenly, add crushed ice, 30% hydrochloric acid solution and sodium nitrite solution. At 15-20°C, PH<...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com