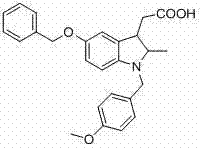
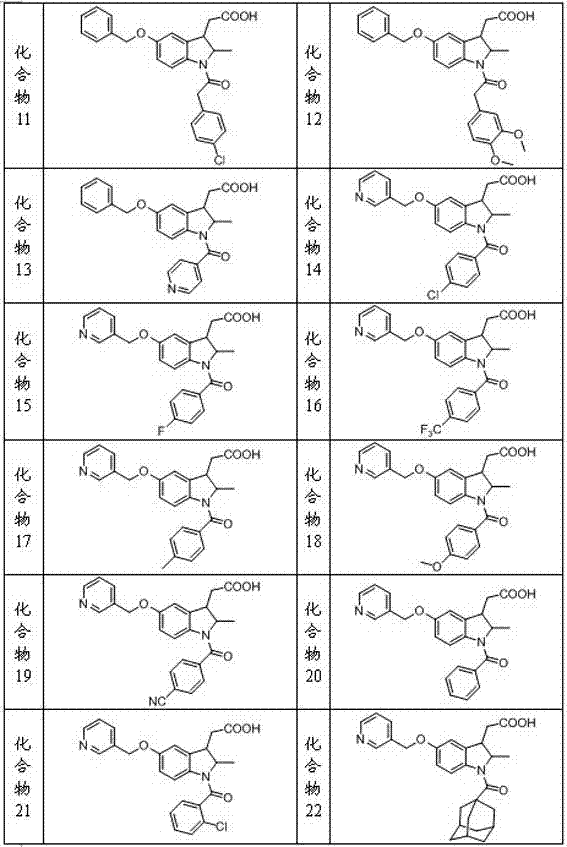
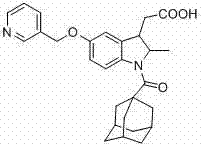
Indoline-3-acetic acid derivative and preparation method thereof as well as application of derivative in medicine
A technology of indoline and derivatives is applied in the application field of indoline-3-acetic acid derivatives in the preparation of anti-type 2 diabetes drugs, and can solve problems such as weight gain, cardiovascular disease and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0081] The preparation method of 2-methyl-1-(4-chlorobenzoyl)-5-benzyloxy-2,3-dihydro-indole-3-acetic acid (compound 1) includes the following steps:
[0082]
[0083] Compound 1 synthetic route
[0084] (1): 2-Methyl-1-(4-chlorobenzoyl)-5-benzyloxy-2,3-dihydro-indole-3-acetic acid methyl ester (1-1)
[0085] 2-Methyl-5-benzyloxy-2,3-dihydro-indole-3-acetic acid methyl ester (0.205g, 0.66mmol) was dissolved in 5ml ethyl acetate and 10ml acetonitrile, and p-chlorobenzene was added to the solution. Formic acid (0.207g, 1.32mmol), 1-ethyl-(3-dimethylaminopropyl)carbodiimide hydrochloride (0.278g, 1.45mmol) N-methylmorpholine (0.16ml, 1.45mmol) ) The reaction was stirred at room temperature for 12 hours. After the reaction, the reaction solution was washed with 1N hydrochloric acid (20ml×3), saturated sodium bicarbonate (20ml×3), distilled water 20ml, saturated sodium chloride 20ml, dried over anhydrous sodium sulfate, and the solvent was evaporated. The residue was passed through a s...
Embodiment 2
[0089] The preparation method of 2-methyl-1-(4-fluorobenzoyl)-5-benzyloxy-2,3-dihydro-indole-3-acetic acid (compound 2) includes:
[0090]
[0091] Using p-fluorobenzoic acid instead of p-chlorobenzoic acid, the title compound was prepared according to the method of Example 1, with a yield of 39.2%. 1 H NMR (CDCl 3 ): δ=1.34 (d, 3H); 2.71 (d, 2H); 3.06 (m, 1H); 4.18 (m, 1H); 5.01 (s, 2H); 6.80 (dd, 1H); 7.90 (d, 1H); 7.36-7.40 (m, 5H); 7.40 (d, 2H); 7.60 (d, 1H); 8.10 (d, 2H). Elemental analysis, C 25 H 22 FNO 4 , Measured value (calculated value), %: C 71.54 (71.59); H 5.23 (5.29); N 3.30 (3.34).
Embodiment 3
[0093] The preparation method of 2-methyl-1-(4-trifluoromethylbenzoyl)-5-benzyloxy-2,3-dihydro-indole-3-acetic acid (compound 3) includes:
[0094]
[0095] Using 4-trifluoromethylbenzoic acid instead of p-chlorobenzoic acid, the title compound was prepared according to the method of Example 1, with a yield of 32.3%. 1 H NMR (CDCl 3 ): δ=1.26 (d, 3H); 2.65 (d, 2H); 3.19 (m, 1H); 4.15 (m, 1H); 5.01 (s, 2H); 6.91 (d, 2H); 7.33-7.40 ( m, 6H); 7.60 (d, 2H); 7.70 (d, 2H). Elemental analysis, C 26 H 22 F 3 NO 4 , Measured value (calculated value), %: C 66.50 (66.52); H 4.69 (4.72); N 2.97 (2.98).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com