Novel enzyme protein, process for production of enzyme protein, and gene encoding enzyme protein
A coding and protein technology, applied in the direction of biochemical equipment and methods, enzymes, transferases, etc., can solve the problems of glycoproteins and glycolipids in reaction results, high and limited specificity of sugar receptor substrates, etc., to achieve easy-to-operate effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0145] Example 1: Production of mutant β-galactoside-α2,3-sialyltransferase
[0146] (1) Production of ISH467-N2C0 mutant
[0147] For β-galactoside-α2,3-sialyltransferase N2C0 derived from Photobacterium phosphoreum JT-ISH-467 strain (Patent Document 3: WO2006 / 112253), mutants were produced in which amino acids were substituted. PCR (1st PCR) was performed using ISH467-N2C0 / pTrc99A (Patent Document 3: WO2006 / 112253) prepared by the inventors as a template. The primer sequences and their combinations used in the reaction are shown in Tables 1 and 2. The 50 μl reaction solution contained plasmid, 5 μl of 10×PyroBest buffer, 4 μl of 2.5 mM dNTP, 5 picomoles of each primer, and 0.5 μl of PyroBest DNA Polymerase (manufactured by TaKaRa Co., Ltd.). , once at 96°C for 3 minutes, 15 times at 96°C for 1 minute, at 55°C for 1 minute, at 72°C for 2 minutes, and once at 72°C for 1 minute (1st PCR). The primer combinations used in PCR are shown in Table 2. These PCR products were s...
Embodiment 2
[0177] Example 2: Activity determination of mutant β-galactoside-α2,3-sialyltransferase
[0178] (1) Determination of sialyltransferase activity of mutant N2C0 containing His×6-Tag
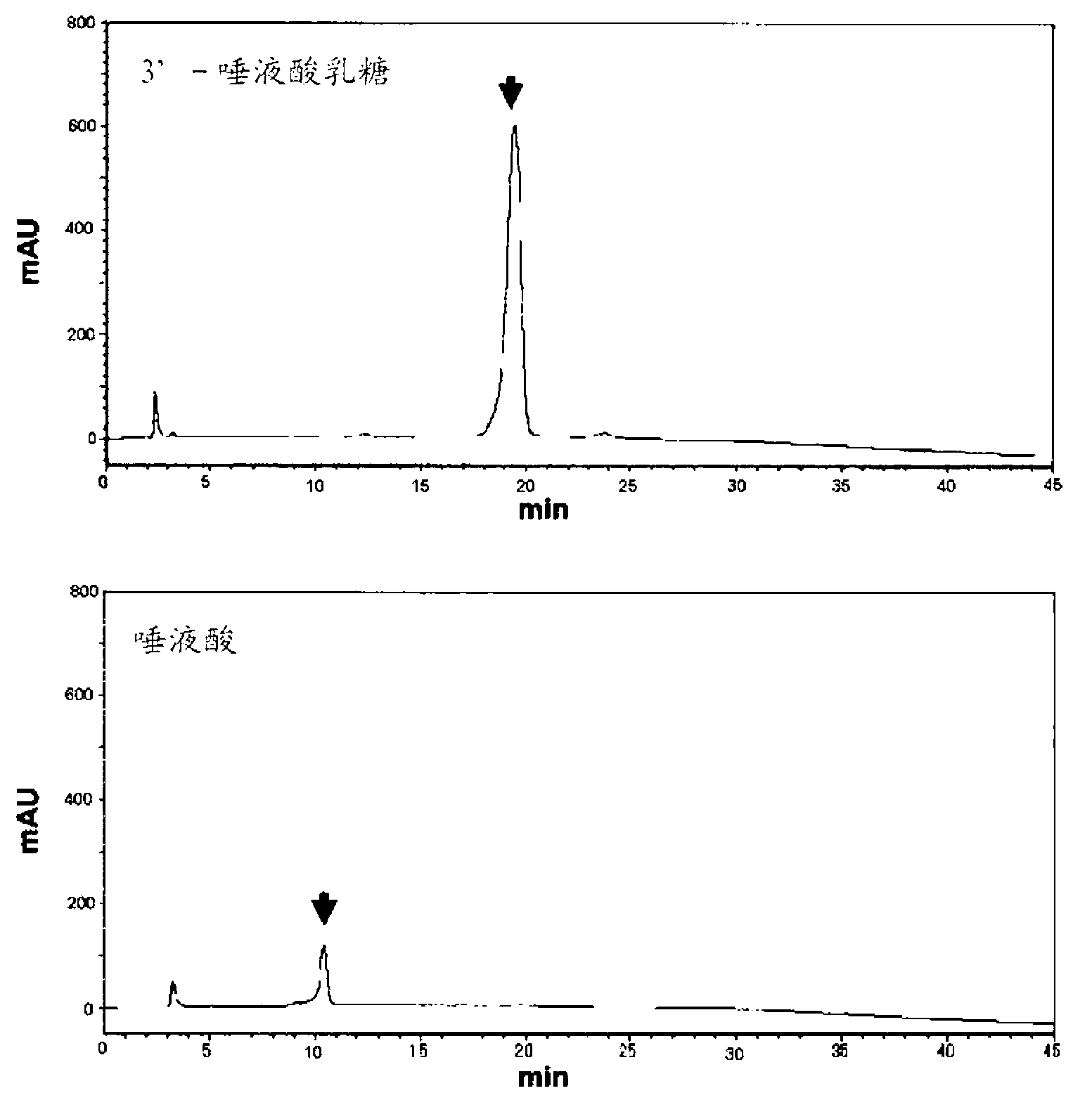
[0179] Regarding the mutant N2CO protein containing His×6-Tag purified in Example 1-(2) (four mutants of F318A, E319A, F318AE319A and S337A), β-galactoside-α2,3-sialic acid Transferase activity assay. The sialic acid transfer activity was determined by the method described in J. Biochem., 120, 104-110 (1996), which is incorporated herein by reference in its entirety. Specifically, the sugar donor substrate CMP-NeuAc (containing 70nmol, 14 C The NeuAc-labeled CMP-NeuAc was 25000 cpm, which was 356 cpm / nmol. NeuAc stands for N-acetylneuraminic acid), lactose (1.25 μmol) as a sugar acceptor substrate, NaCl was added to a concentration of 0.5M, and the reaction solution (30 μl) containing the enzyme prepared by the method described above was used for the enzyme reaction. The enzyme reaction w...
Embodiment 3
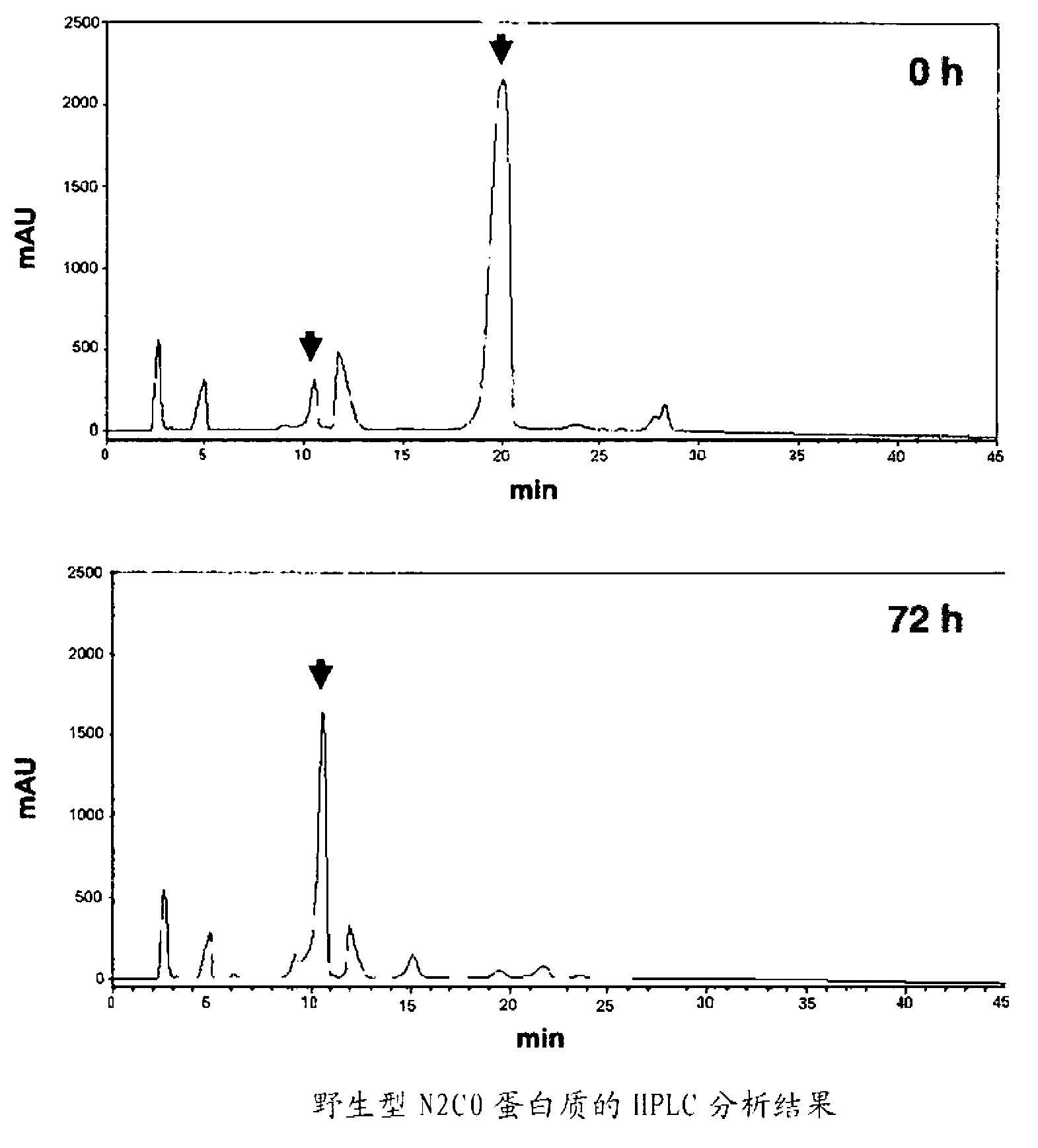
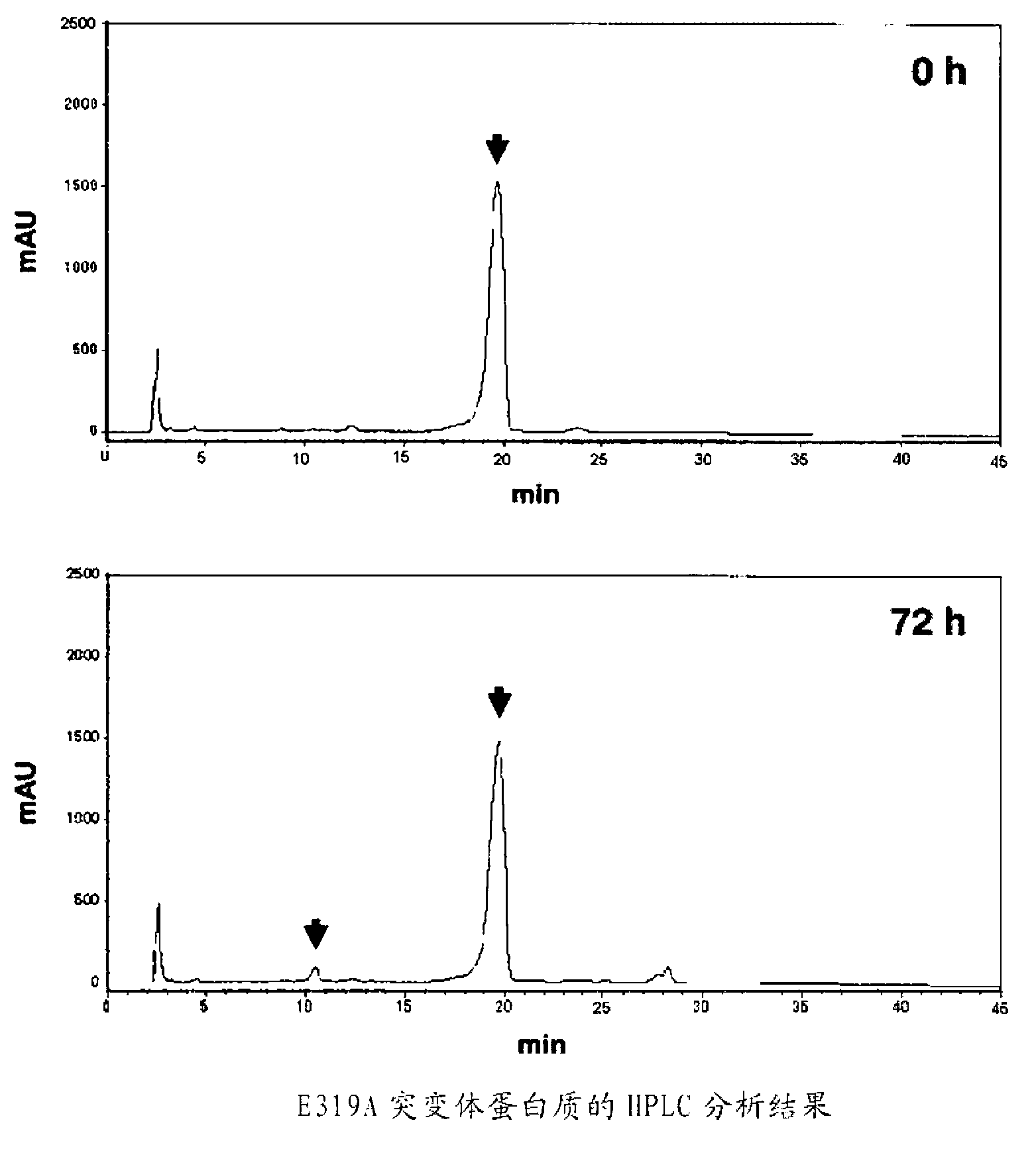
[0185] Example 3: Properties of modified β-galactoside-α2,3-sialyltransferase
[0186] Using the modified N2C0 protein without His × 6-Tag purified in Example 1-(4) (two kinds of E319A, F318AE319A) and the wild-type N2C0 protein without His × 6-Tag, calculate their relative lactose and CMP-sialic acid rate constants (Table 3). Km and V of ISH467-N2C0 without His×6-Tag relative to lactose and CMP-sialic acid max They are 8.4mM and 0.72mM respectively. Km and V of ISH467-N2C0 mutants E319A and F318AE319A without His×6-Tag relative to lactose and CMP-sialic acid maxThey are 22mM, 0.53mM and 85mM, 1.59mM respectively.
[0187] [Table 3] Table 3 Modified and wild-type N2C0 protein relative to the rate constant of lactose and CMP-sialic acid
[0188] lactose
[0189]
K m (mM)
V max (nmol / min)
k cat ( / Second)
N2CO enzyme
8.4
3.9
11.2
E319A mutant enzyme
22
12
99.7
F318AE319A mutant enzyme
85
7.2...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com