Application of ubiquitin-binding domain fusion protein in detection and separation of ubiquitination protein
A technology for ubiquitinated proteins and binding domains, applied in chemical instruments and methods, biochemical equipment and methods, enzymes, etc., can solve the problems of poor ubiquitination detection effect and low sensitivity of protein ubiquitination detection, Achieve high detection capacity and sensitivity, low cost, and improve efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
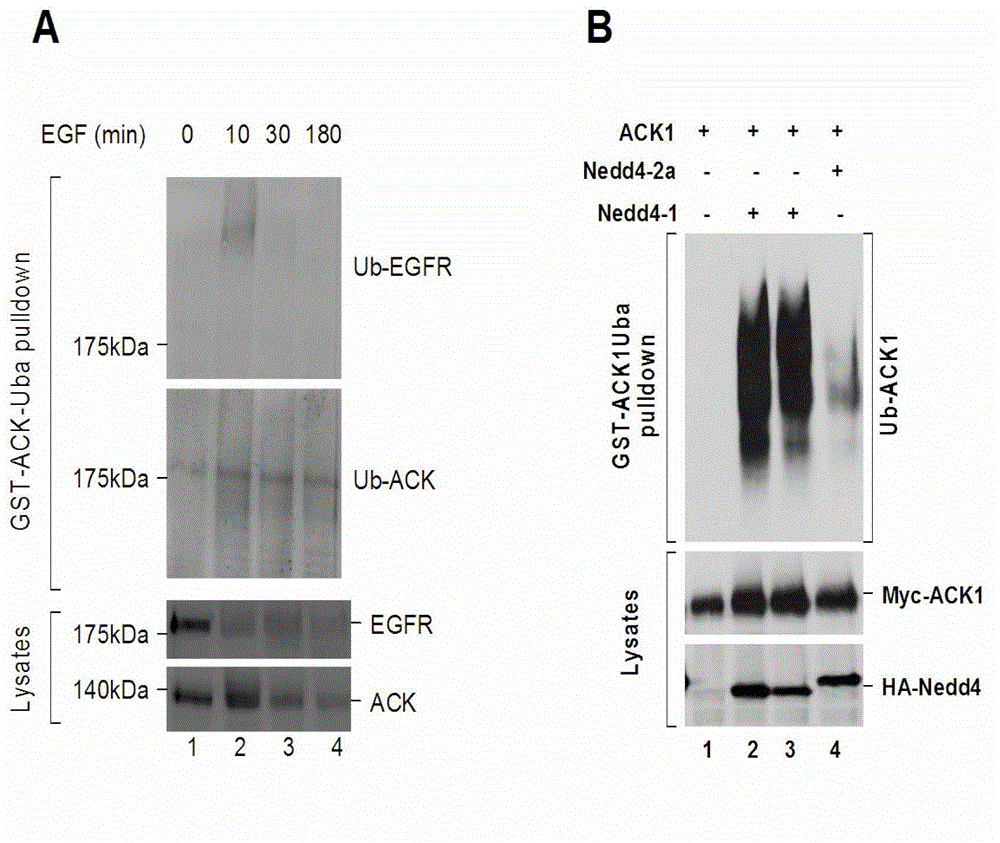
[0018] Example: Isolation and detection of ubiquitinated proteins using a fusion protein of GST-ACK1Uba.
[0019] 1. Expression and purification of GST-ACK1Uba fusion protein.
[0020] ACK1 (Activated Cdc42-associated Kinase1, also known as Tnk2) is a non-receptor tyrosine kinase whose carboxyl terminus contains a UBA domain. The ACK1UBA domain was cloned into the GST fusion protein expression vector pGEX4T3 (GE Life Sciences) to obtain the plasmid pGEX4T3-GST-ACK1Uba. The sequence of the fusion protein cDNA plasmid was confirmed by DNA sequencing. pGEX4T3-GST-ACK1Uba was transformed into E. coli JM109 for expression of the fusion protein. The transformed bacteria were cultured in LB medium at 37°C to a density of A600 (light absorption at 600nm) of 1.0, and then 1 / 1000 IPTG (0.5mM) was added to induce fusion protein expression for 3-5 hours. The bacteria after fusion protein expression were collected by centrifuging at 8000 rpm for 10 minutes.
[0021] The pelleted bacter...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com