Antimicrobial formulation
An antimicrobial, compositional technology, applied in the direction of biocides, chemicals for biological control, animal repellants, etc., that can solve problems such as prevention of mold growth that does not indicate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
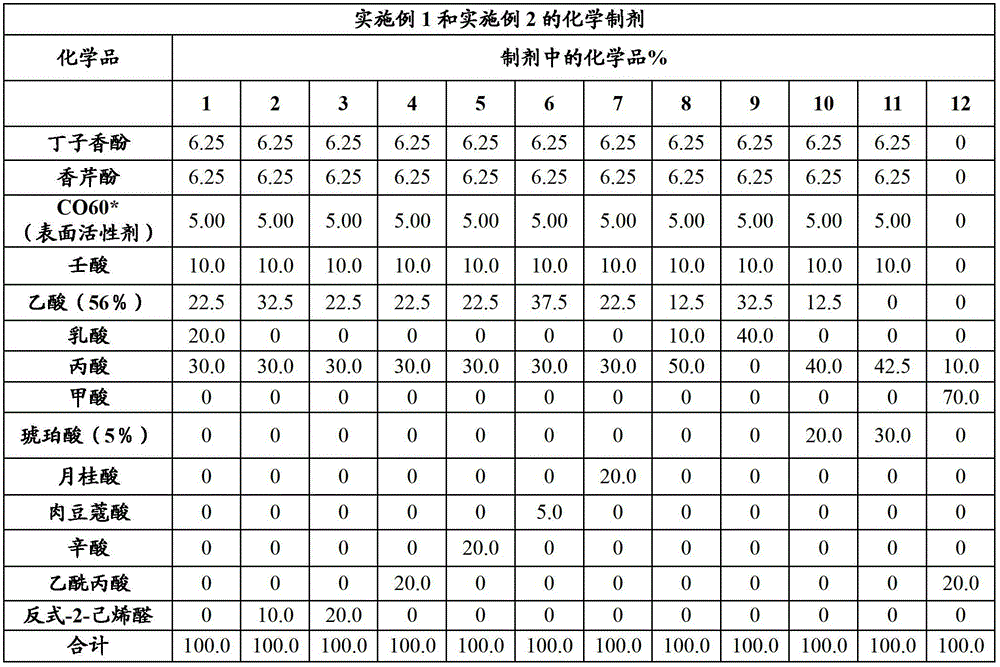
Embodiment 1 and 2
[0088] Procedure: The following formulations were prepared for repeat in vitro studies. All reagents are of the highest purity and laboratory grade. For acetic acid, a 56% solution was prepared. Due to solubility issues, dilute succinic acid to a 5% solution in water. For comparison purposes, two commercial products, a formic acid / propionic acid blend and a formaldehyde / propionic acid blend, were tested.
[0089]
[0090] *CO-60 is an ethoxylated castor oil surfactant with 60 ethylene units.
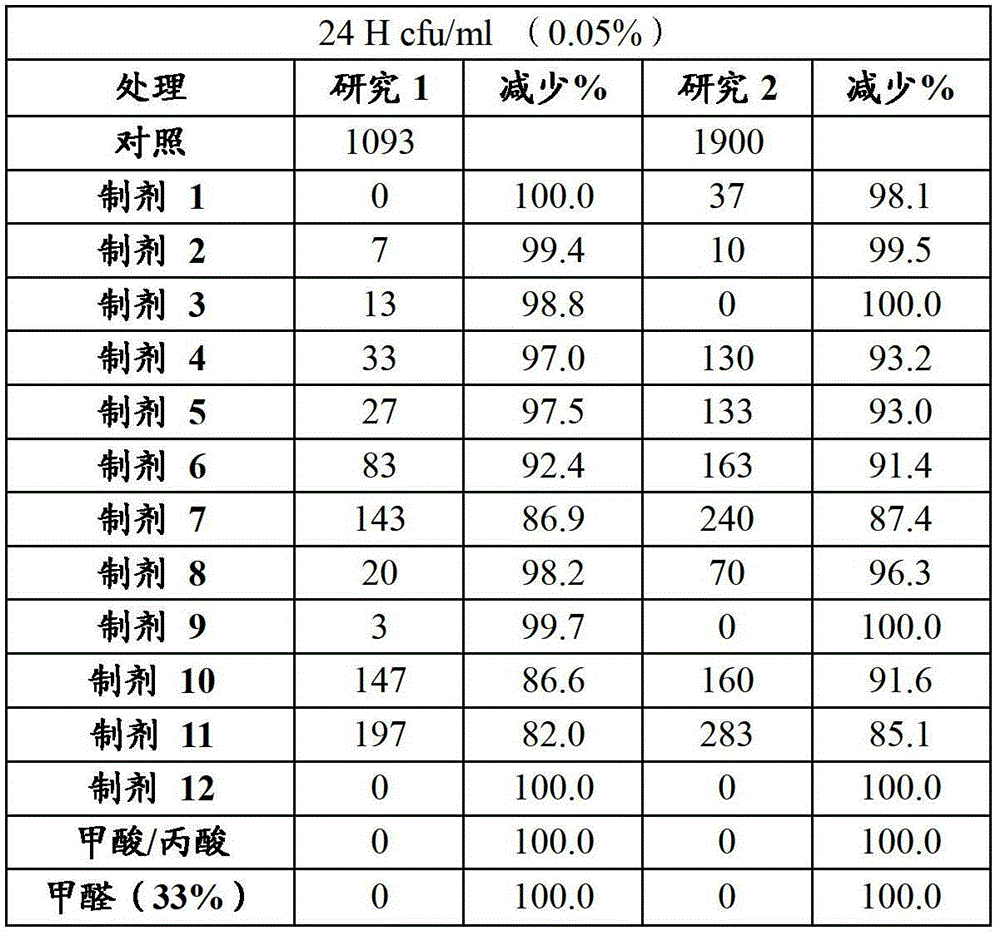
[0091] A suspension of S. typhimurium was added to two tubes containing 0.05% (500 ppm) of each formulation. The tubes were vortexed and incubated for 24 hours at room temperature, then the solution was plated on SMA (Standard Method Agar) for 48 hours before counting Salmonella colonies.
[0092] Results: The following table shows that several formulations were effective in controlling the growth of Salmonella.
[0093] Results: Study 1 and Study 2
[0094]
[0095] Conclusi...
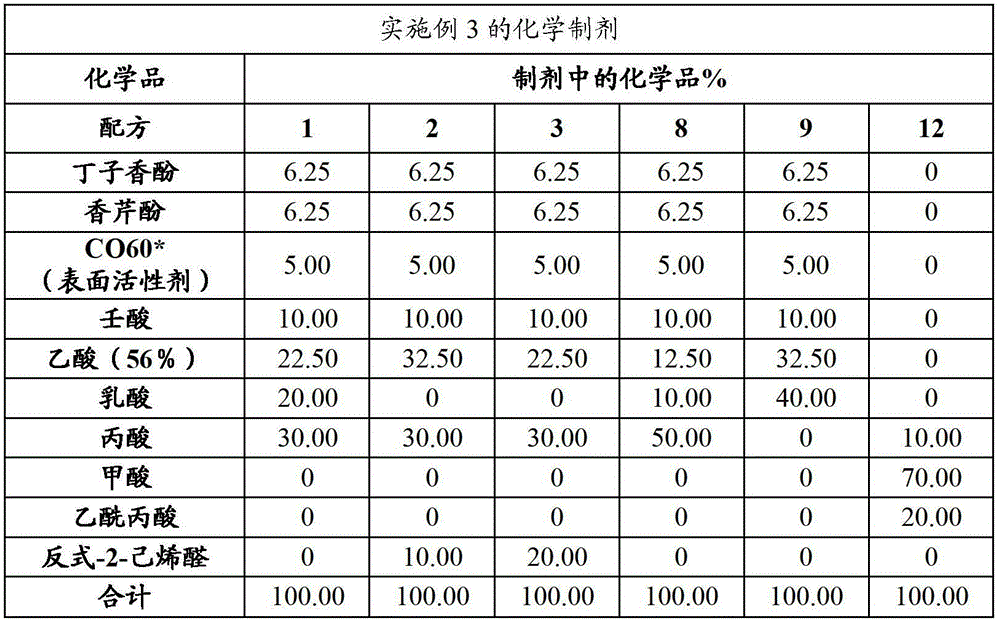
Embodiment 3
[0097] Procedure: From previous in vitro studies, six formulations were selected to test their effectiveness against Salmonella in feed. For comparison purposes, a formaldehyde / propionic acid blend was tested. Poultry meal feed was modified with a meat and bone meal inoculum of S. typhimurium. The contaminated feed was then treated with one of the following formulations at 1 kg / MT, 2 kg / MT and 3 kg / MT. After 24 hours, a subsample of 10 g of the treated feed was suspended in 90 ml of Butterfield's buffer. Dilutions were plated on XLT-4 agar and incubated at 37°C for 48 hours before counting Salmonella colonies. The formulations used in this experiment are shown in the table below.
[0098]
[0099] *CO-60 is an ethoxylated castor oil surfactant with 60 ethylene units.
[0100] Results: The table below shows that several formulations were effective in controlling the growth of Salmonella.
[0101] deal with
[0102] Conclusions: Formulations containing trans-2...
Embodiment 4
[0104] Procedure: Five formulations were selected to test their effectiveness against S. typhimurium in feed. Modification of poultry meal feed with a meat and bone meal inoculum of Salmonella typhimurium. The contaminated feed was then treated with one of the following formulations at 1 kg / MT, 2 kg / MT and 3 kg / MT. After 24 hours, a subsample of 10 g of the treated feed was suspended in 90 ml of Butterfield's buffer. Dilutions were plated on XLT-4 agar and incubated at 37°C for 48 hours before counting Salmonella colonies. Additional samples were taken 7 and 14 days after Salmonella enumeration treatment. The formulations used are shown in the table below.
[0105]
[0106] *CO-60 is an ethoxylated castor oil surfactant with 60 ethylene units.
[0107] Results: The table below shows that several formulations were effective in controlling Salmonella.
[0108]
[0109] Conclusions: All formulations resulted in a reduction in the number of Salmonella in the feed. Form...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com