Repaglinide and metformin hydrochloride medicinal composition and its preparation method
A technology for metformin hydrochloride and a composition is applied in the field of pharmaceutical compositions containing repaglinide and metformin hydrochloride and the field of preparation thereof, which can solve the problems of complicated preparation process and high production cost, and achieve good fluidity, low friability, Good tablet hardness
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1-4
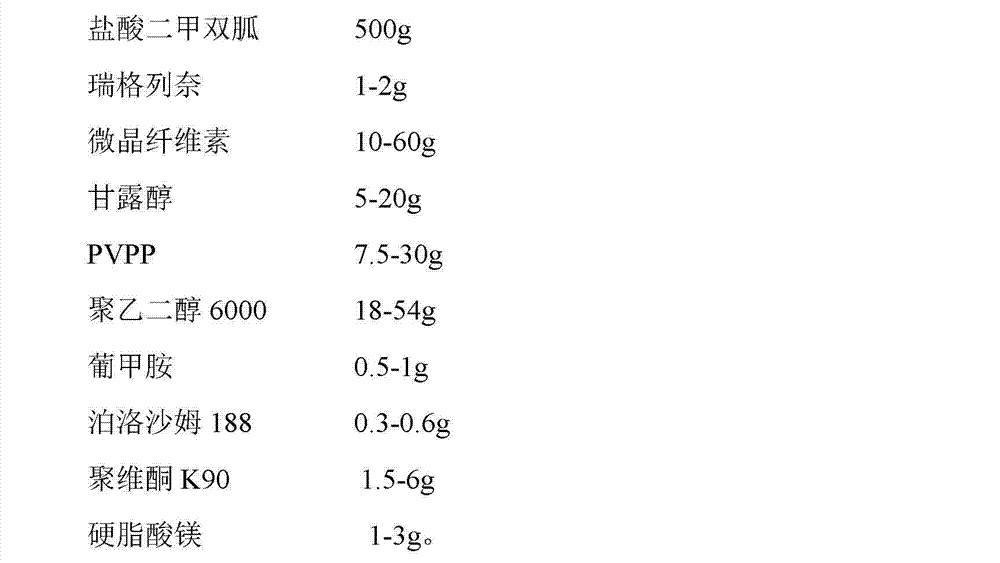
[0047] Embodiment 1-4, tablet
[0048] The preferred formulation of the pharmaceutical composition of the present invention is as follows: prescription (quantity of 1000 tablets, unit: g)
[0049]
[0050] The composition preparation method of the present invention comprises the following steps:
[0051] a) Dissolving poloxamer and meglumine in 50% ethanol, then adding repaglinide, and then adding povidone after dissolving;
[0052] b) Metformin hydrochloride is pulverized through a 60-mesh sieve, and mannitol is passed through a 30-mesh sieve for subsequent use;
[0053] c) Weigh the prescribed amount of metformin hydrochloride, microcrystalline cellulose, crospovidone, mannitol and polyethylene glycol 6000 and put them in the fluidized bed, and set the air intake volume to 500±200m 3 / h, air inlet temperature 90±5℃, product temperature 70±5℃, hot melt granulation;
[0054] d) Spray the repaglinide solution into the fluidized bed, set the atomization pressure to 1.0±0.2...
Embodiment 5-6
[0059] Embodiment 5-6, tablet of the present invention
[0060]
[0061] The composition preparation method of the present invention comprises the following steps:
[0062] a) Dissolving poloxamer and meglumine in 50% ethanol, then adding repaglinide, and then adding povidone after dissolving;
[0063] b) Metformin hydrochloride is pulverized through a 60-mesh sieve, and mannitol is passed through a 30-mesh sieve for subsequent use;
[0064] c) Weigh the prescribed amount of metformin hydrochloride, microcrystalline cellulose, crospovidone, mannitol and polyethylene glycol 6000 and put them in the fluidized bed, and set the air intake volume to 500±200m 3 / h, air inlet temperature 90±5℃, product temperature 70±5℃, hot melt granulation;
[0065] d) Spray the repaglinide solution into the fluidized bed, set the atomization pressure to 1.0±0.2bar, and the spray speed to 30±10Hz, and perform one-step granulation;
[0066] e) passing the prepared granules through a 1.0mm roun...
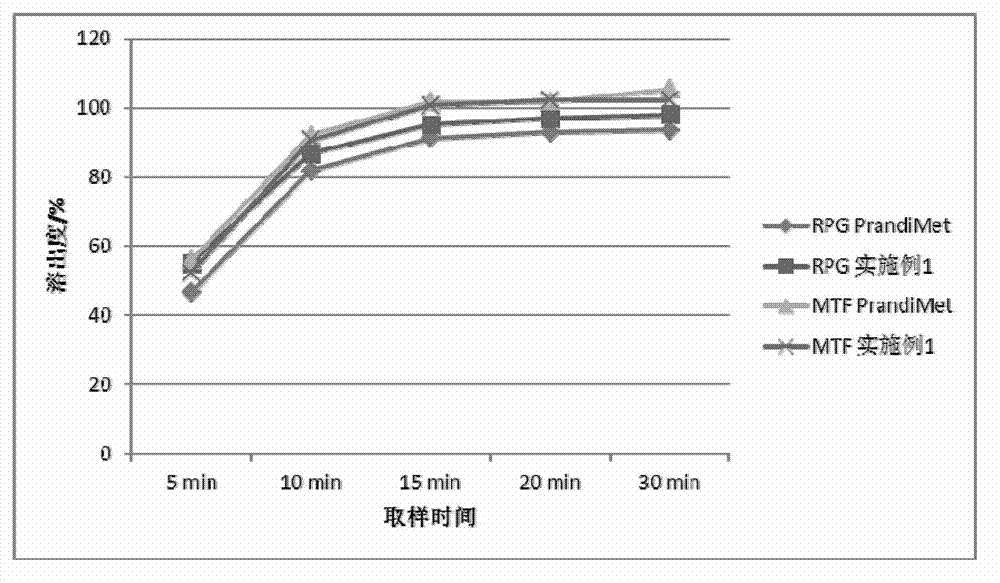
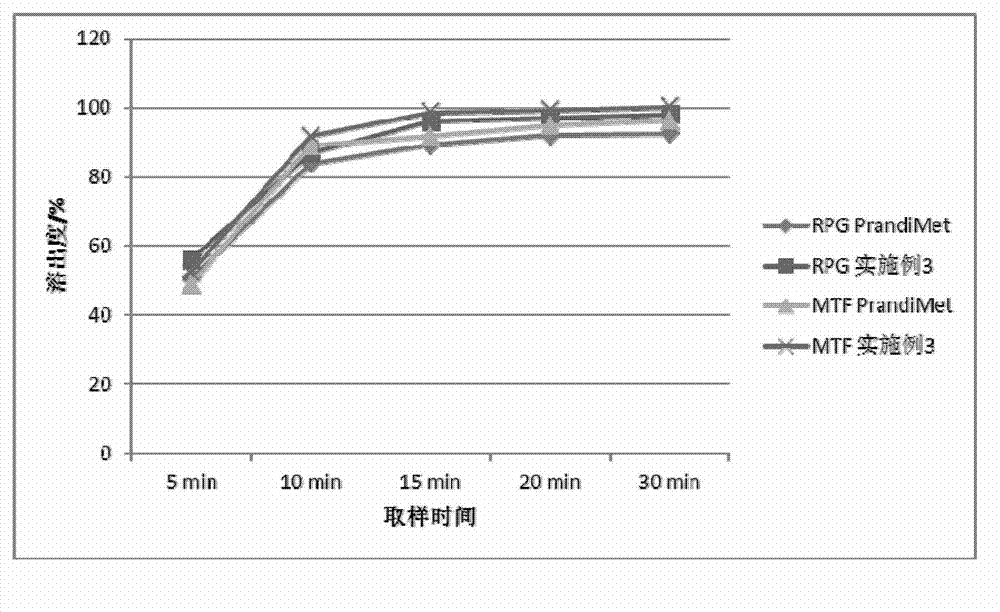
experiment example 1
[0070] Experimental example 1, the physical property determination of granule of the present invention and tablet
[0071] Get the granule and tablet of above-mentioned embodiment 1-6, carry out granule fluidity, tablet hardness, friability test
[0072] The results of particle fluidity (angle of repose), bulk density, tapped density, tablet hardness and friability are shown in the table below:
[0073]
[0074] From the results in the table above, it can be seen that the fluidity of the granules of Examples 1-6 is not much different, the tablet hardness is suitable, and the friability is small.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com