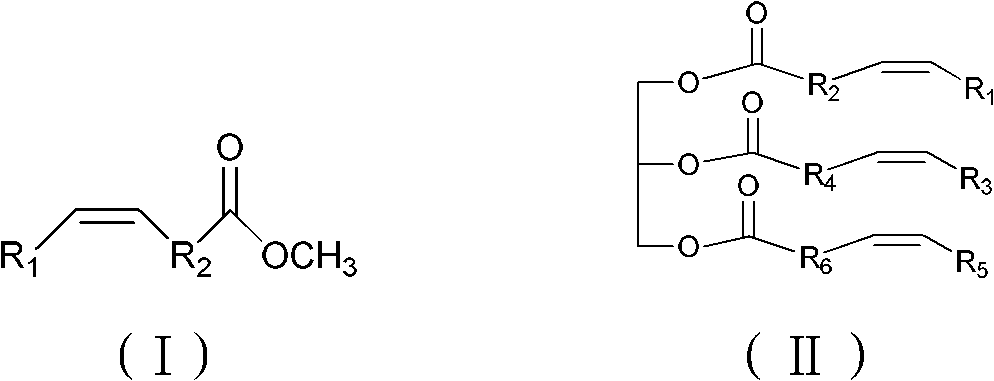
Synthesizing method of epoxy fatty acid ester
A technology of epoxy fatty acid ester and synthesis method, applied in the direction of organic chemistry, etc., can solve the problems of discontinuous and unsafe processes, and achieve the effects of eliminating cold spots and hot spots, improving safety and rapid control.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
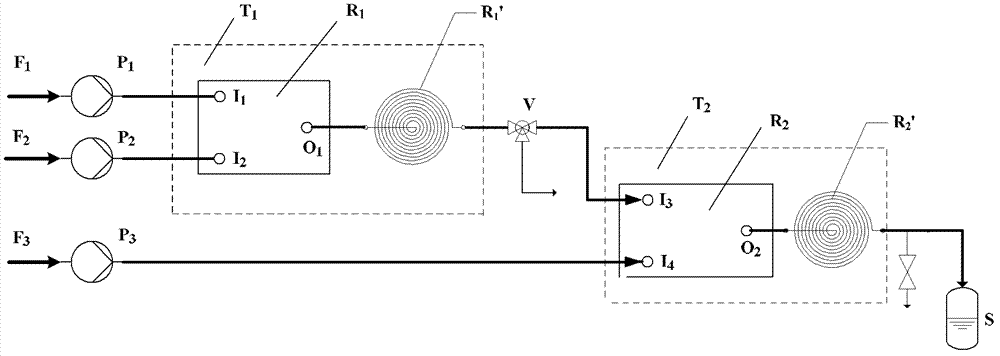
[0027] Use constant flow pump P 1 ,P 2 (Series II digital pump, Chrom Tech, Inc.) to control formic acid feedstock F 1 and hydrogen peroxide raw material F 2 Output, make 50wt.% (catalyst is sulfuric acid 5wt.%, water 45wt.%) hydrogen peroxide solution and the molar ratio of anhydrous formic acid are that the flow of 1.5: 1 passes into microreactor 1 (R 1 ) formic acid inlet I 1 with hydrogen peroxide inlet I 2 , contact and initiate the peroxidation reaction in the first microreactor, so that the initiated peroxidation reaction material is in contact with the microreactor R 1 Exit O 1 Connected capillary extension 1 (R 1 ') to continue the reaction, mixing and reaction total time is 10 minutes, generating peroxyformic acid, by temperature control system T 1 -The temperature of the microreactor 1 and the capillary extension tube 1 is controlled by a constant temperature water bath to 40°C, which is approximately the temperature of the peroxidation reaction, and the conc...
Embodiment 2
[0038] Embodiment 2, microreactor 2 channel dimensions change
[0039] According to the same method as in Example 1, the difference from Example 1 is that a microreactor with a hydraulic diameter of 0.5 mm is used to replace the microreactor 2, i.e. the epoxidation microreactor, and other experimental conditions are the same as those in Implementation 1. The product was analyzed, and the iodine value Iv was 5.8, and the epoxy value Oe was 5.3%. The calculated double bond conversion rate X of soybean oil methyl ester was 95.4%, and the epoxy selectivity S was 69%. It can be seen that the slight adjustment of the scale of the reaction equipment will not have a great impact on the reaction results.
Embodiment 3
[0040] Embodiment 3, hydrogen peroxide concentration and reaction time change
[0041] According to the same method of Example 1, the difference from Example 1 is that increasing the concentration of hydrogen peroxide is 70wt.%, catalyst sulfuric acid 5wt.%, water 25wt.%, and anhydrous formic acid in a microreactor with a molar ratio of 1.5: 1 1 and the contact reaction in the extension tube 1 for 5 minutes, the concentration of peroxyformic acid generated is 1.6mol L -1 be 2.1: 1 to react 5min in microreactor 2 and extension tube 2 with soybean oil methyl ester by the mol ratio of the double bond in the initial hydrogen peroxide and soybean oil methyl ester, and reaction temperature is raised to 70 ℃, and the ring that analyzes obtains The fatty acid ester product was oxidized, and the iodine value was 40.1, and the epoxy value was 5.1%. The calculated double bond conversion rate of soybean oil methyl ester is 68.2%, and the epoxy selectivity is 92%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com