Plant in-vitro ubiquitin protein degradation system and application thereof
A plant protein, ubiquitin protein technology, applied in the field of plant in vitro ubiquitin protein degradation system, can solve the problems of single, false positive, false negative and so on
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0089] Example 1. Construction of recombinant expression vector
[0090] 1. Preparation of recombinant plasmid pET28a-UBA2
[0091] 1. Design primers to amplify the full CDS sequence of Arabidopsis ubiquitin activating enzyme UBA2 gene (GenBank: U40566.1, nucleotide sequence from 414 to 3647 at the 5'end, sequence 2) and separate at both ends Add EcoRI and SalI restriction sites.
[0092] 2. Use the primers of step 1 to clone the CDS full sequence of UBA2 gene by PCR, and cut with restriction enzymes EcoRI and SalI.
[0093] 3. Cut the pET28a vector with restriction enzymes EcoRI and SalI to obtain the vector backbone.
[0094] 4. Connect the digested product of step 2 with the vector backbone of step 3 to obtain the recombinant plasmid pET28a-UBA2. Sequencing showed that the recombinant plasmid pET28a-UBA2 was inserted between the EcoRI and SalI sites of the backbone vector pET28a: GenBank: U40566.1, from the 5'end 414 to 3647 nucleotide sequence, that is, the sequence 2 in the seque...
Embodiment 2
[0149] Example 2. Preparation of protein
[0150] 1. Expression and purification of 6×His fusion protein and UBCH5b protein
[0151] 1. Preparation of E. coli Bl21 (DE3) competent cells.
[0152] 2. The recombinant plasmids prepared in step 1 and step 6 of Example 1 with pET28a as the vector backbone were respectively heat shocked into B121 (DE3) competent cells.
[0153] 3. Pick 2-3 single clones in 3-10 mL of LB liquid medium supplemented with corresponding antibiotics, and cultivate overnight at 37°C at 200 rpm.
[0154] 4. Add the cell culture after overnight to 200-500mL LB liquid medium containing the corresponding antibiotics and 0.2% (0.2g / 100mL) glucose to make the OD600 about 0.1.
[0155] 5. When shaking culture at 18°C until the OD600 is 0.5-0.6, add the inducer isopropyl-β-D-thiogalactopyranoside (IPTG) to a final concentration of 0.2mM; induce at 18°C for 12-16h.
[0156] 6. Centrifuge for 10 minutes at 4°C, 4000 rpm, and collect the bacteria.
[0157] 7. Pre-cooled Lysis...
Embodiment 3
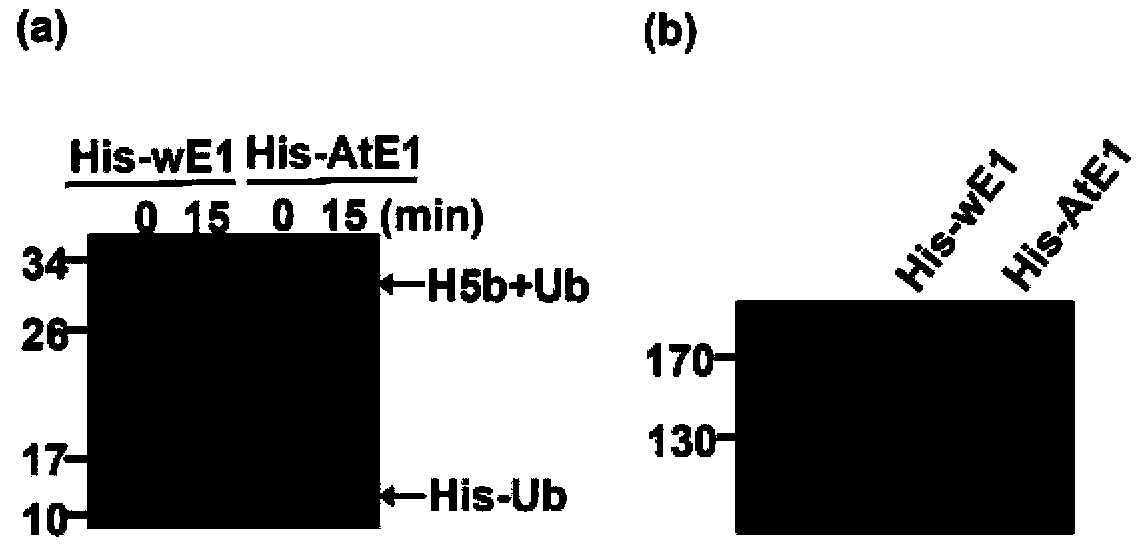
[0180] Example 3, Example 2 Purified Arabidopsis ubiquitin activating enzyme and Arabidopsis ubiquitin protein activity detection
[0181] In this example, the activity of the Arabidopsis ubiquitin activating enzyme and the Arabidopsis ubiquitin protein purified in Example 2 was tested according to the following method:
[0182] (1) Take two 1.5mL Eppendorf tubes and mark them as sample 1 and sample 2. Add 0.2μg of human ubiquitin conjugating enzyme (UBCH5b) obtained in Example 2, 3-5μg of Arabidopsis wild-type monomeric ubiquitin protein (His-Ub) and 1μL of 20× reaction buffer ( Formula: 1M Tris pH7.5, 100mMATP, 50mM MgCl 2 , 2mM DTT, solvent is water). In addition, 0.05μg of Arabidopsis ubiquitin activating enzyme UBA2 (His-AtE1) was added to sample 1, and the same amount of wheat ubiquitin activating enzyme (His-wE1) was added to sample 2. Add ddH to both samples 2 O to a total volume of 20 μL.
[0183] (2) After mixing the samples, take out 1 / 2 volume of the reaction solution,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com