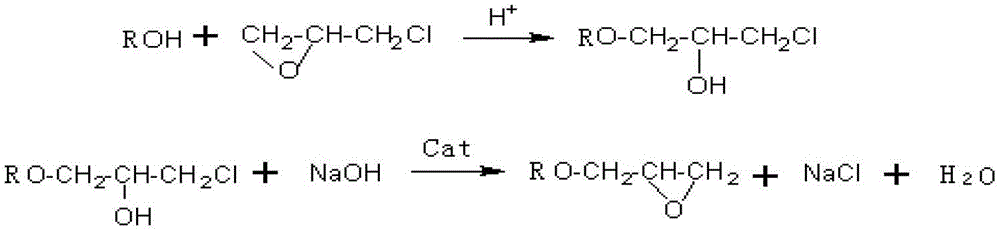
Synthetic method of butyl glycidyl ether
A technology of glycidyl ether and synthesis method, applied in the direction of organic chemistry, etc., which can solve the problems of corrosion of reaction equipment, low epoxy value of the final product, and inability to recycle and use
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] Take 5g of activated carbon and impregnate in 75g of HNO with a mass fraction of 10-30% 3 In the solution, the immersion temperature is 20-40°C, the immersion time is 3-4h, then the activated carbon is taken out and washed to neutrality, filtered and dried to obtain the modified activated carbon; after the modified activated carbon is impregnated in the The catalyst can be obtained by immersing in 75g of toluene solution with 0.5% boron mass fraction, immersing temperature at 30-45°C, and immersing time for 3-4 hours, filtering and drying. The immobilized amount of the activated carbon-supported boron trifluoride catalyst was measured to be 5%.
Embodiment 2
[0024] Take 5g of activated carbon and first impregnate it in 50g of HNO with a mass fraction of 10-30%. 3 In the solution, the immersion temperature is 40-70°C, the immersion time is 2-3h, then the activated carbon is taken out and washed to neutrality, filtered and dried to obtain the modified activated carbon; after the modified activated carbon is impregnated in the The catalyst can be obtained by immersing in 50g of toluene solution with 2% boron mass fraction, immersing temperature at 45-60°C and immersing time for 2.5-3 hours, filtering and drying. It is measured that the immobilized amount of the activated carbon-supported boron trifluoride catalyst is 16%.
Embodiment 3
[0026] Take 5g of activated carbon and impregnate in 25g of HNO with a mass fraction of 10-30% 3 In the solution, the immersion temperature is 70-100°C, the immersion time is 1-2h, then the activated carbon is taken out and washed to neutrality, filtered and dried to obtain the modified activated carbon; after the modified activated carbon is impregnated in the The catalyst can be obtained by immersing in 50g of toluene solution with 5% boron mass fraction at 60-70°C for 1-2.5 hours, filtering and drying. It is measured that the immobilized amount of the activated carbon immobilized boron trifluoride catalyst is 20%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| epoxy value | aaaaa | aaaaa |
| epoxy value | aaaaa | aaaaa |
| epoxy value | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com