Application of flavonoid in pharmacy
A technology of flavonoids and drugs, applied in the application field of subsidence, can solve the problem of not using daily life and so on
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
experiment example 1
[0113] Experimental Example 1: Control Experiment of Staphylococcus aureus Stimulating Human Neutrophils to Synthesize and Release Histamine
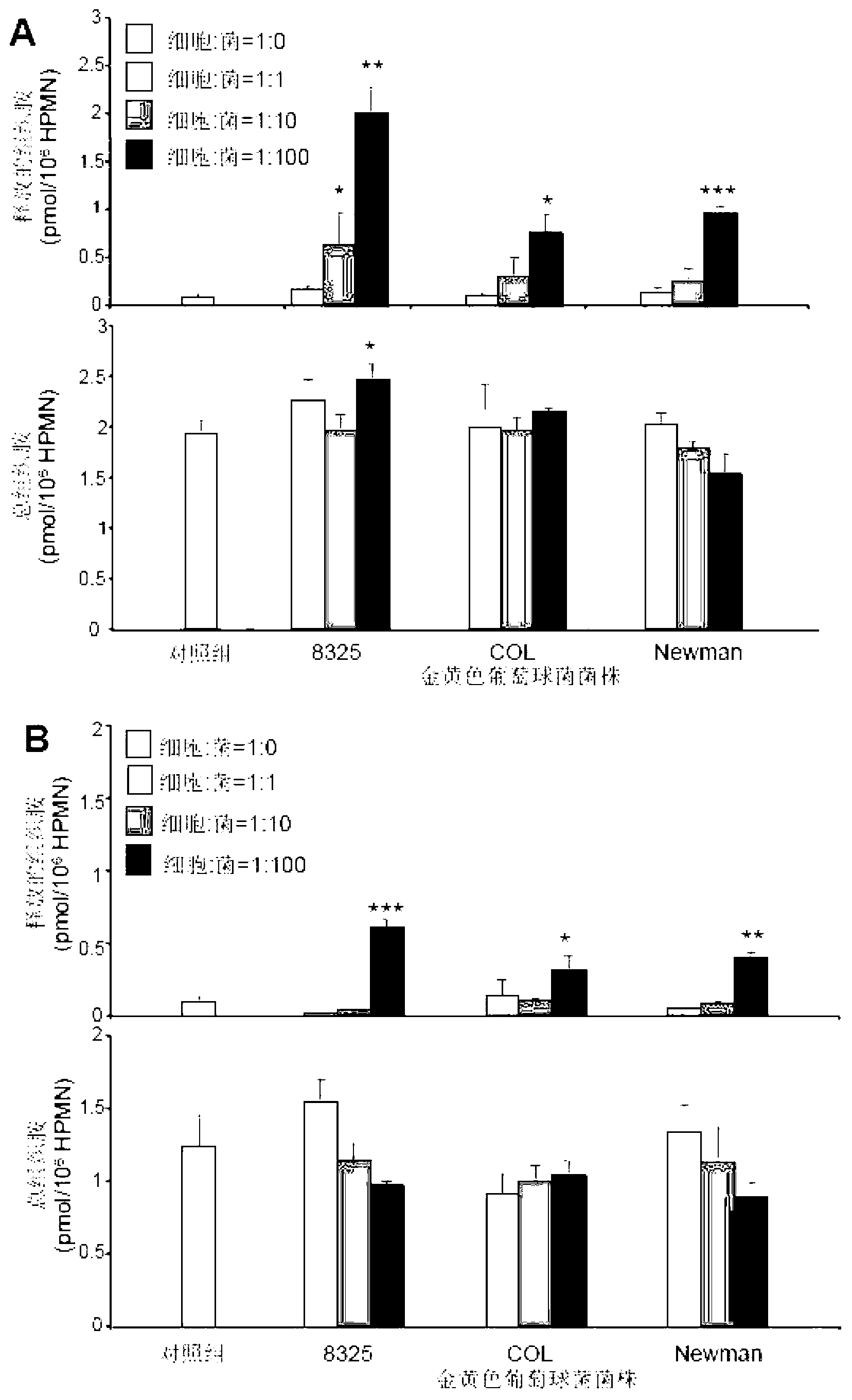
[0114] see figure 1 , Staphylococcus aureus stimulates human neutrophils to synthesize and release histamine. different concentrations of live ( figure 1 A) or heat-killed ( figure 1 B) Staphylococcus aureus can stimulate human neutrophils (referred to as "HPMN", the same below) to release histamine (the concentration refers to the ratio of the number of cells to the number of bacteria, abbreviated as "cell: bacteria"; the control group refers to is the neutrophil group without bacteria, the same as the other legends below). This experiment was improved by the method reported previously (Xu Xiang et al., Journal of Leukocyte Biology 2012; 91:275-284), the same below. The control cells themselves produced and released a small amount of histamine. Some cells in the human body can release a small amount of histamine under normal phy...
experiment example 2
[0115] Experimental Example 2: Control Experiment of Staphylococcus aureus Stimulating Human Neutrophils to Synthesize and Release Leukotriene B4
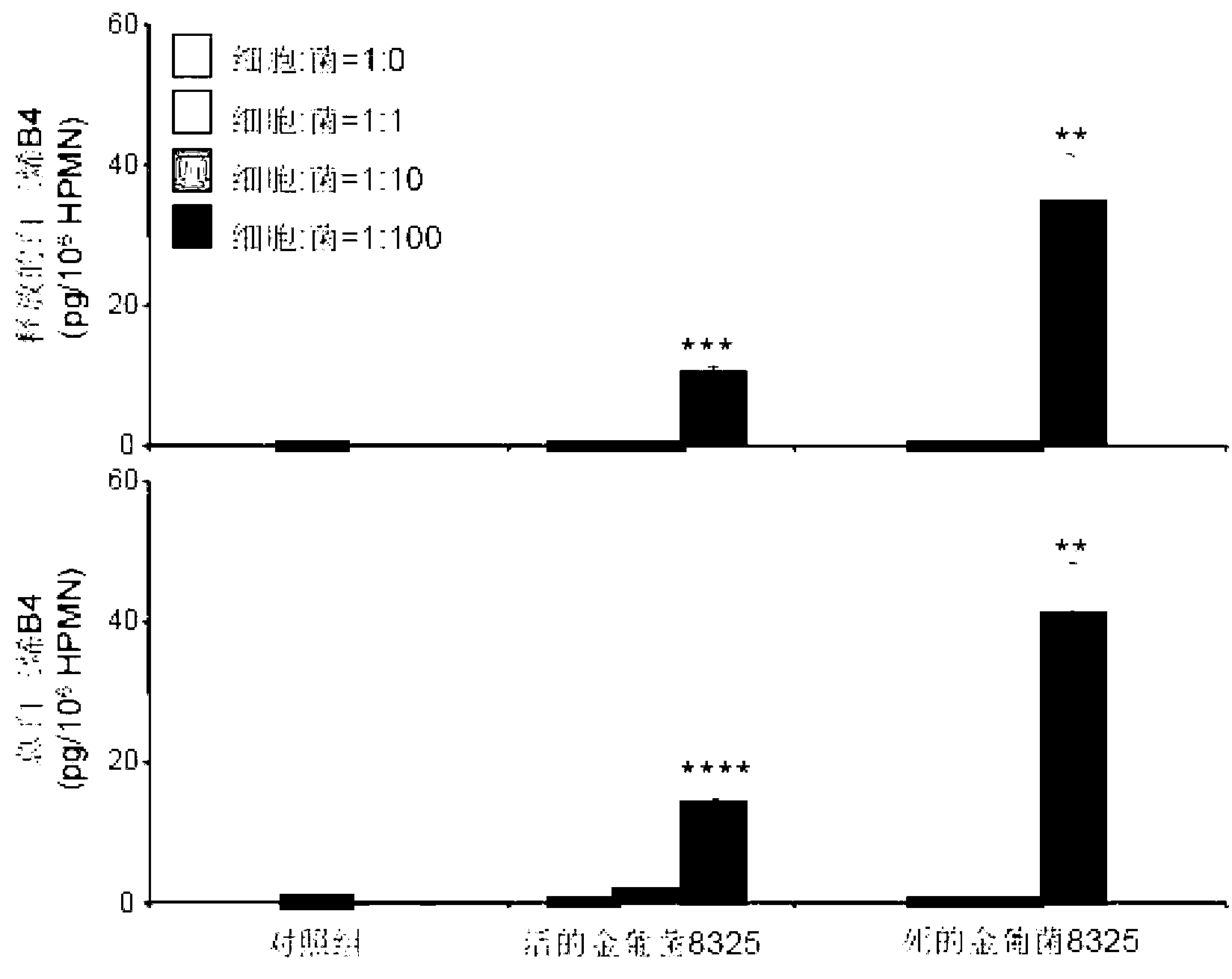
[0116] see figure 2 , the 8325 strain was used in this experiment. Different concentrations of live or heat-killed S. aureus can stimulate the production and release of leukotriene B4 from human neutrophils. The control cells themselves also produced and released very small amounts of leukotriene B4. And dead Staphylococcus aureus can stimulate human neutrophils to synthesize and release more leukotriene B4. Compared with the control group, a high concentration (cell:bacteria=1:100) of dead S. aureus can stimulate cells to newly synthesize more than 63 times of leukotriene B4 and release more than 84 times of leukotriene B4. (**p<0.01, ****p<0.0001, compared to the control group).
experiment example 3
[0117] Experimental example 3 : Control experiments of flavonoids inhibiting the synthesis and release of histamine in human neutrophils stimulated by live or dead Staphylococcus aureus
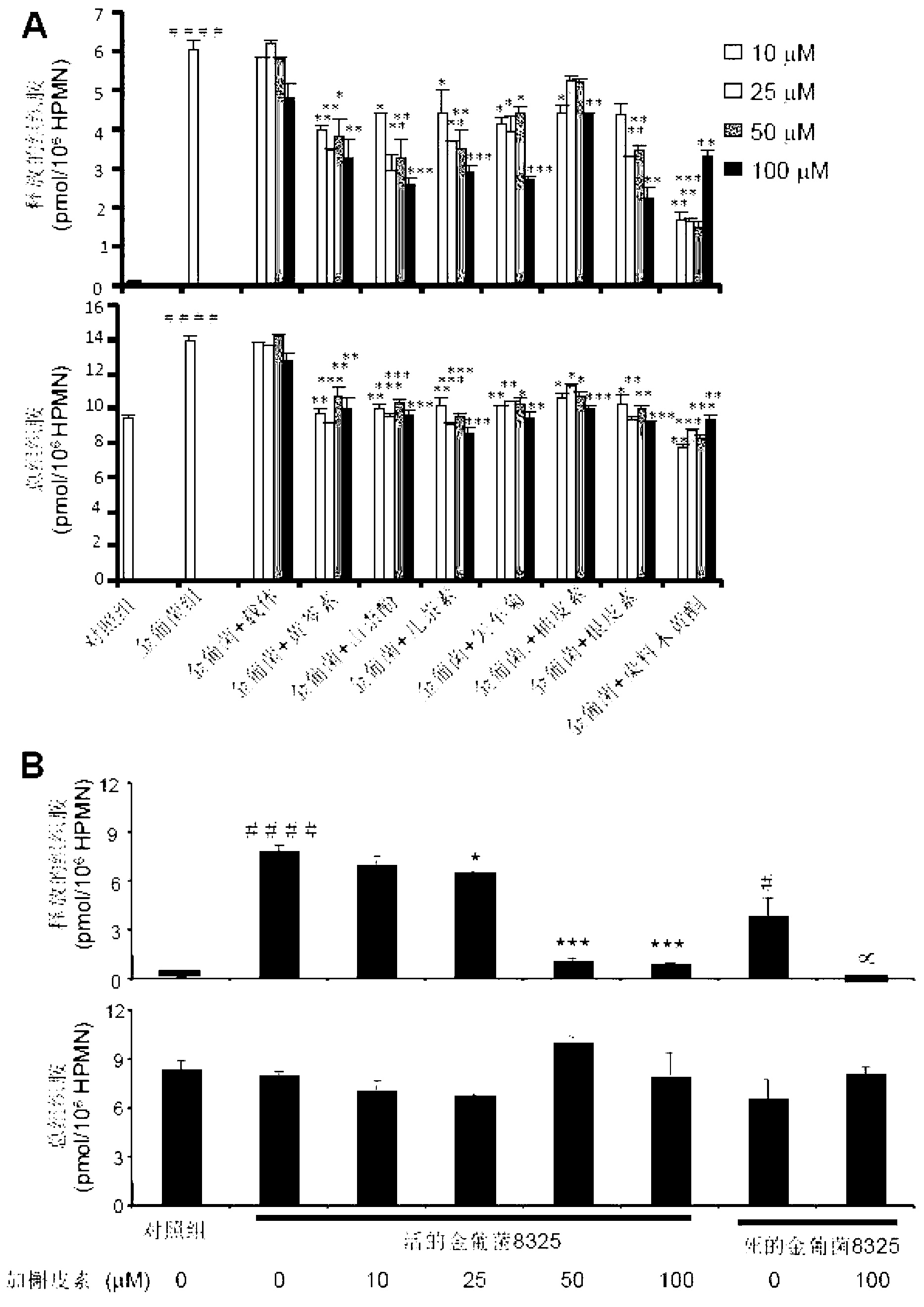
[0118] see image 3 , The 8325 strain was used in this experiment, and the ratio of cells to Staphylococcus aureus was 1:100. image 3 A, shows that the S. aureus group without flavonoids can significantly stimulate the production and release of histamine from human neutrophils compared with the control group (the newly synthesized histamine increased by 1.5 times compared with the control group and released 65.6-fold increase in histamine). Seven flavonoids at different concentrations can inhibit the production and release of histamine by human neutrophils stimulated by Staphylococcus aureus. % (25mM naringenin group) to more than 75% (50mM genistein group), while the inhibition rate of newly synthesized histamine by S. aureus stimulated cells ranged from 24.3% (10mM naringenin group) ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com