Temperature and ion dual-sensitive in-situ gel nasal cavity drug delivery system
A temperature-sensitive, in-situ gel technology, used in pharmaceutical formulations, medical preparations with inactive ingredients, organic active ingredients, etc., can solve the problem of short duration of action on nasal mucosa, unfavorable drug effect, and easy loss of sprays, etc. problem, to achieve the effect of favorable drug absorption, good drug sustained release, and good sprayability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Example 1. Preparation of ion-sensitive nasal in situ gel
[0036]Weigh an appropriate amount of gellan gum, add an appropriate amount of deionized water to the beaker, fully swell in a hot water bath at 70-80°C, and then obtain a clear solution in a refrigerator at 4°C, and prepare the quality of gellan gum respectively Gel matrix with fractions of 0.2%, 0.5%, 0.75%, 1.0% (w / v), in a constant temperature water bath at 36.5°C, mixed with artificial nasal fluid (v / v=1:1) to observe each gel The gel condition of the matrix, the results are shown in Table 1.
[0037] Table 1 Gel conditions after mixing different mass fractions of gellan gum and artificial nasal solution
[0038]
[0039] The results show that when gellan gum is simply used as the in-situ gel matrix for nasal use, the gel cannot be formed at all when the concentration is too low; if the concentration is too high, the fluidity is poor and nasal spray administration cannot be performed. Therefore, it n...
Embodiment 2
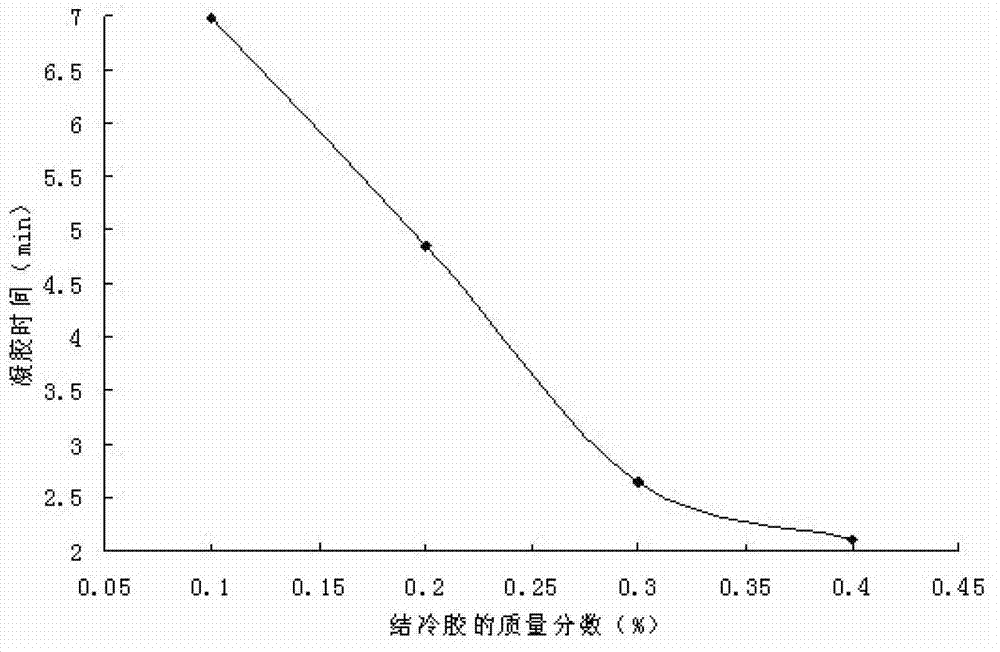
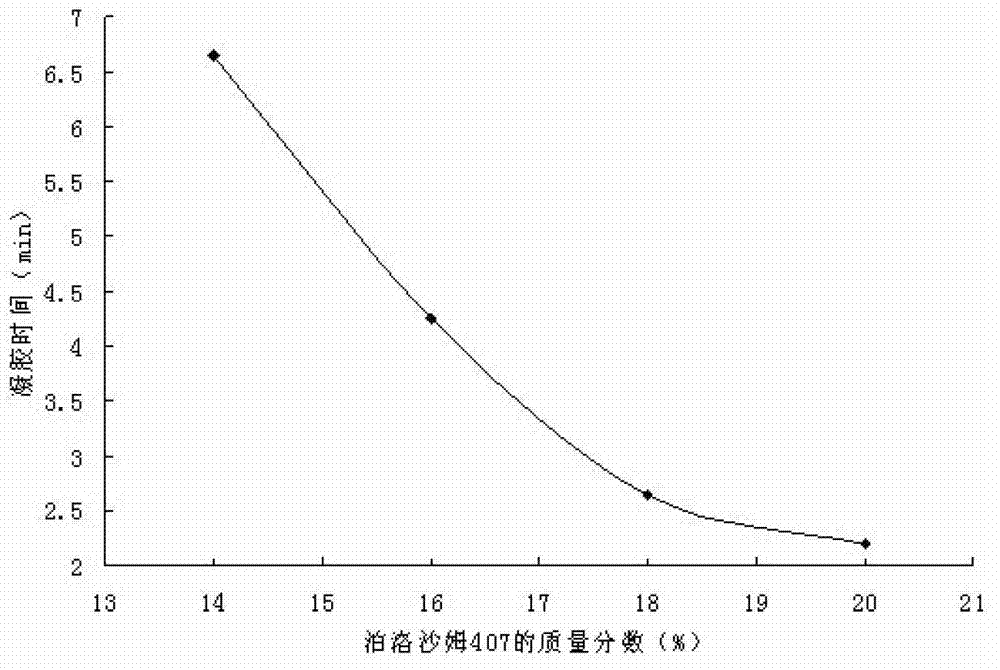
[0040] Example 2. Changes in gelation time of composite in situ gels with different concentrations
[0041] Take an appropriate amount of gellan gum in a beaker, add an appropriate amount of deionized water, fully swell in a hot water bath at 70-80°C, and then place it in a refrigerator at 4°C to obtain a clear solution; another appropriate amount of poloxamer 407, Add it to the above solution, stir evenly, and place it at 4°C for 48 hours to prepare a composite gel. Prepare 0.1% gellan gum + 18% poloxamer 407, 0.2% gellan gum + 18% poloxamer 407, 0.3% gellan gum + 18% poloxamer 407, 0.4% gellan gum + 18% Poloxamer 407, 14% Poloxamer 407+0.3% Gellan Gum, 16% Poloxamer 407+0.3% Gellan Gum, 18% Poloxamer 407+0.3% Gellan Gum, Composite gel of 20% poloxamer 407+0.3% gellan gum, V 复合型凝胶 :V 人工鼻液 =4:1, in a 36.5°C constant temperature water bath, investigate the gelation time of each composite gel, the results are shown in Table 2.
[0042] Table 2 The gelation time of differen...
Embodiment 3
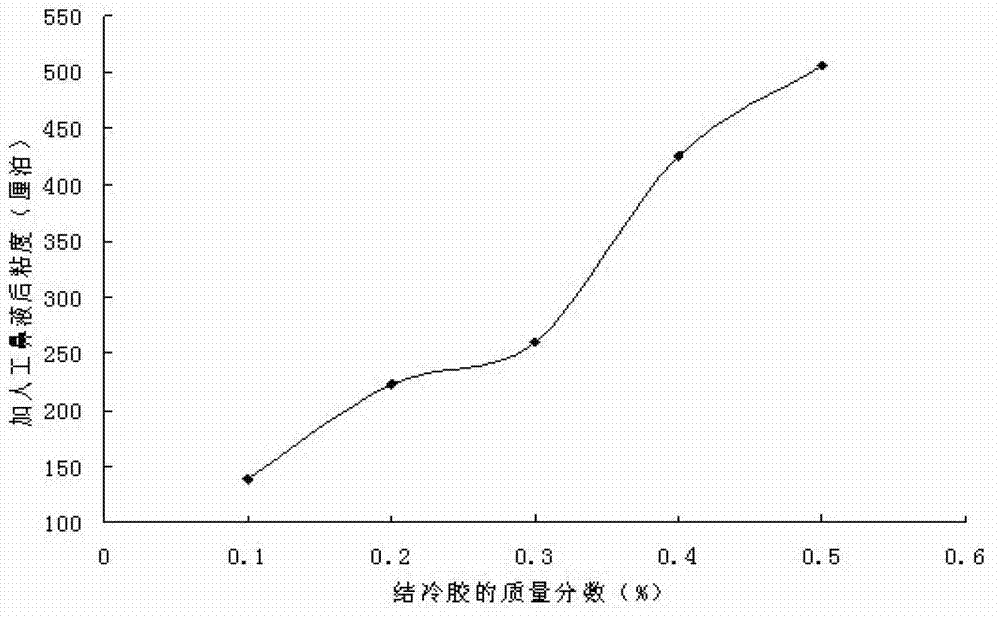
[0048] Example 3. Changes in the viscosity of the composite in situ gel at different concentrations
[0049] Mix the composite gel with the artificial nasal fluid, and use a thermometer to control the reaction temperature to 36.5°C under a circulating water bath, and measure its viscosity value with RVDVIII (rotational viscometer). See image 3 . With the increase of gellan gum solution concentration, the in situ gel viscosity increased significantly.
[0050] See Figure 4 . With the increase of poloxamer 407 concentration, the in situ gel viscosity increased significantly.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com