Sodium fusidate freeze-dried powder injection and preparation method thereof
A technology of sodium fusidate and freeze-dried powder injection, which is applied in the field of pharmaceutical preparations to achieve the effects of reducing content, improving stability, and facilitating safe use
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] Embodiment 1: Preparation of sodium fusidate freeze-dried powder injection of the present invention
[0023] 1. Preparation method
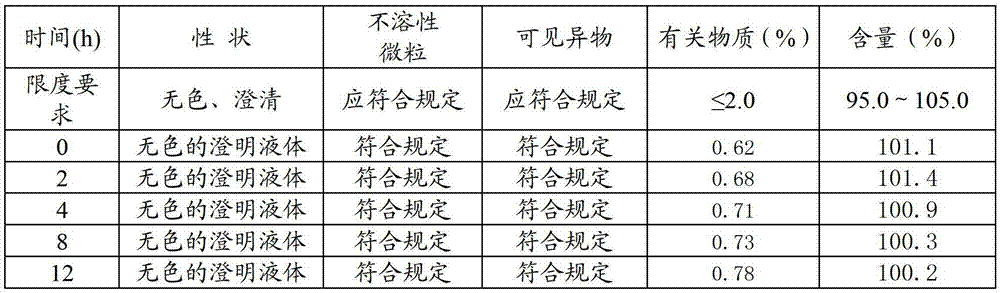
[0024] Weigh 500 parts of sodium fusidate, 20 parts of dimercaptopropanol, 10 parts of erythorbic acid, 15 parts of cysteine hydrochloride, and 10 parts of phenylalanine, and stir and dissolve with 80% of the total amount of water for injection below 35 °C , then lower the temperature to room temperature, add and dissolve 500 parts of sodium fusidate under stirring, then add arginine (25 parts) to adjust the pH value to 8.6, then add the remaining water for injection, then add activated carbon and stir, filter and decarbonize Finally, put the filtrate into a freeze-drying box, freeze it to below -35°C, keep it warm for more than 1.5 hours, turn on the vacuum pump, sublimate at 0°C until the ice line disappears, then raise the temperature to 30°C, keep it warm for 12 hours and dry to obtain sodium fusidate Freeze-dried powder injection, ...
Embodiment 2
[0038] Embodiment 2: Preparation of sodium fusidate freeze-dried powder injection of the present invention
[0039] 1. Preparation method
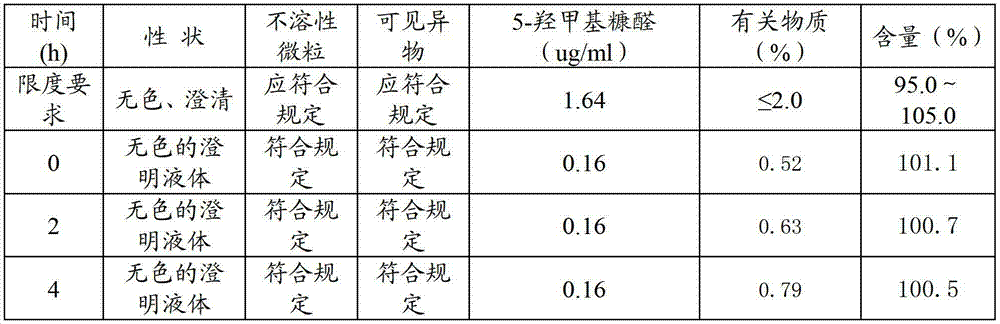
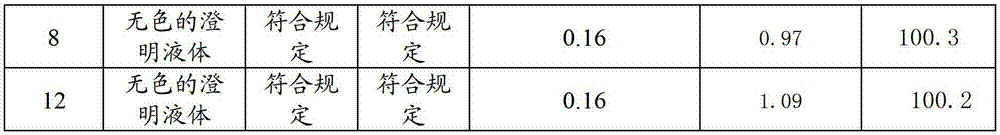
[0040] Weigh 500 parts of sodium fusidate, 20 parts of dimercaptopropanol, 10 parts of erythorbic acid, 15 parts of cysteine hydrochloride, and 10 parts of phenylalanine, and stir and dissolve with 80% of the total amount of water for injection below 35 °C , then lower the temperature to room temperature, add and dissolve 500 parts of sodium fusidate under stirring, then add arginine (40 parts) to adjust the pH value to 8.7, then add the remaining water for injection, then add activated carbon and stir, filter and decarbonize Finally, put the filtrate into a freeze-drying box, freeze it to below -35°C, keep it warm for more than 1.5 hours, turn on the vacuum pump, sublimate at 0°C until the ice line disappears, then raise the temperature to 30°C, keep it warm for 12 hours and dry to obtain sodium fusidate Freeze-dried powder injection, ...
Embodiment 3
[0054] Embodiment 3: Preparation of sodium fusidate freeze-dried powder injection of the present invention
[0055] 1. Preparation method
[0056] Weigh 500 parts of sodium fusidate, 20 parts of dimercaptopropanol, 10 parts of erythorbic acid, 15 parts of cysteine hydrochloride, and 10 parts of phenylalanine, and stir and dissolve with 80% of the total amount of water for injection below 35 °C , then lower the temperature to room temperature, add and dissolve 500 parts of sodium fusidate in a stirring state, then add arginine (65 parts) to adjust the pH value to 8.9, then add the remaining water for injection, then add activated carbon and stir, filter and decarbonize Finally, put the filtrate into a freeze-drying box, freeze it to below -35°C, keep it warm for more than 1.5 hours, turn on the vacuum pump, sublimate at 0°C until the ice line disappears, then raise the temperature to 30°C, keep it warm for 12 hours and dry to obtain sodium fusidate Freeze-dried powder inject...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com