Catalyst for preparing synthetic natural gas and preparation method thereof
A technology for synthesizing natural gas and catalysts, applied in chemical instruments and methods, physical/chemical process catalysts, metal/metal oxide/metal hydroxide catalysts, etc., can solve poor selectivity, high cost, and easy carbon deposition of Fe-based catalysts Inactivation and other problems, to achieve the effect of activity and stability, easy production, high selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
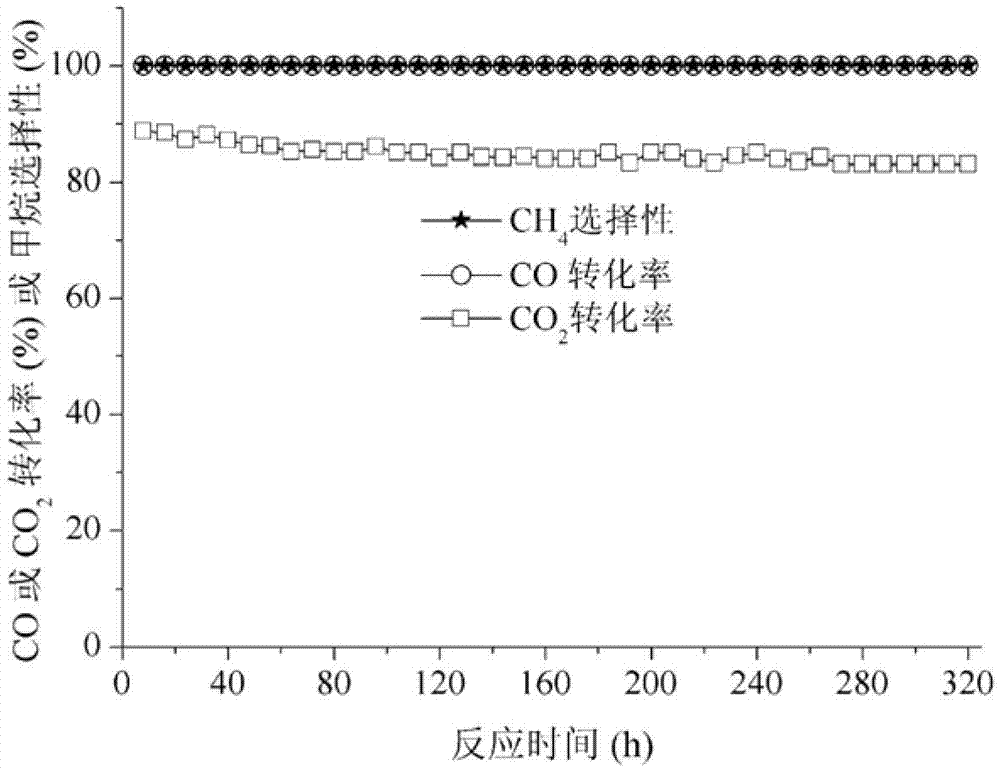
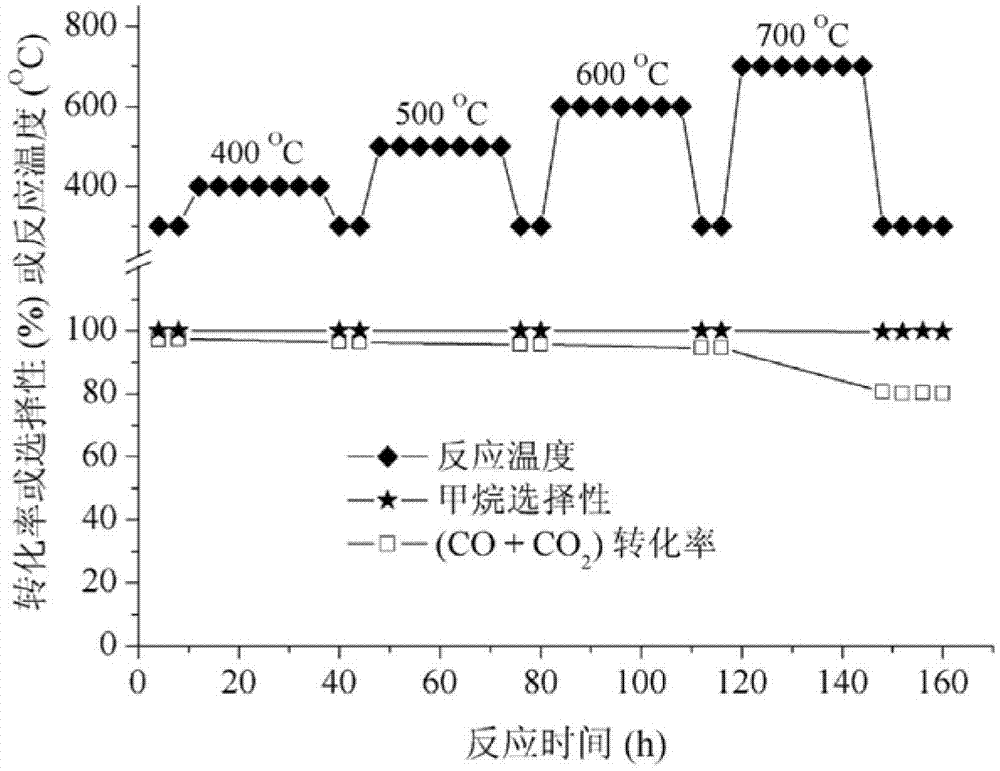
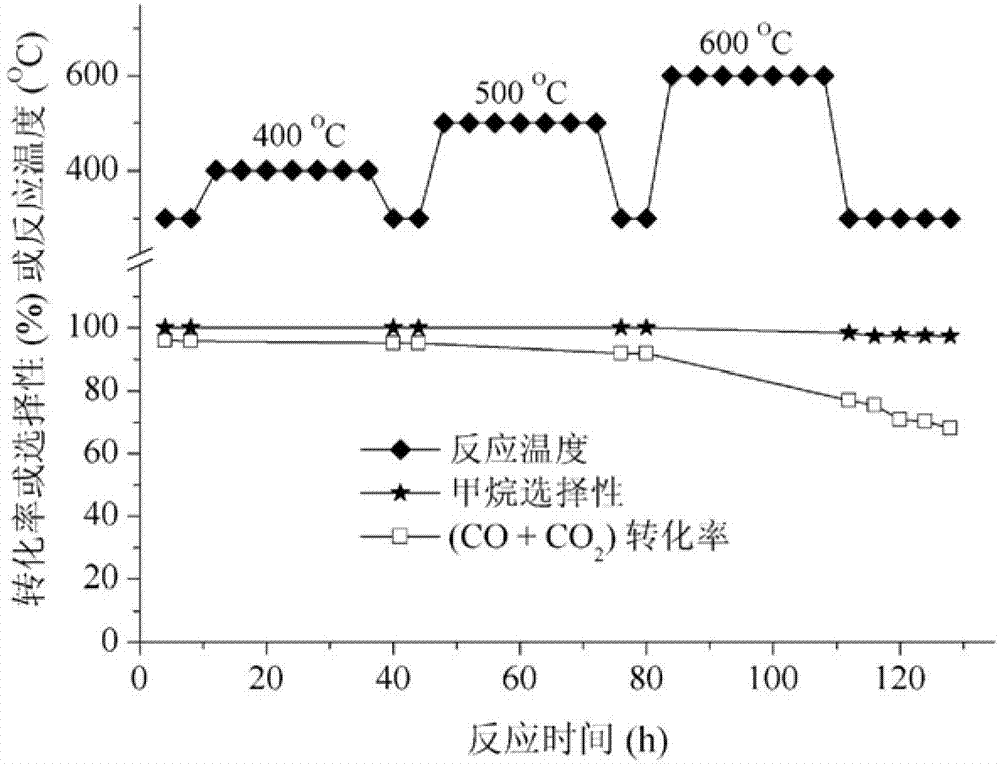
Image
Examples
Embodiment 1
[0022] 6.979gNi(NO 3 ) 2 ·6H 2 O, 4.719g Zr(NO 3 ) 4 ·3H 2 O and 0.924g Sc(NO 3 ) 3 (Purities are all AR grade) mixed together, add 90mL deionized water to make solution A; another 7.837g AR grade anhydrous K 2 CO 3 Dissolve in 95mL deionized water to prepare solution B; at 80°C, inject solution A and solution B into the reaction vessel at an equal velocity (injection rate: 12mL / min), and carry out at a constant temperature of 80°C and constant stirring. coprecipitation reaction, by regulating the K 2 CO 3 Add the amount of aqueous solution to keep the pH value of the precipitation solution at 7.3 to 7.6. After the addition, continue to stir for 30 minutes and then stop stirring. Immediately filter the feed solution, and the obtained precipitate is washed with deionized water until it reaches K in the eluent. + The ion concentration is below 0.1ppm (detected by flame ion absorption method), after filtration, the filter cake is dried at 110°C for 18h, and roasted at 4...
Embodiment 2
[0030] 6.979gNi(NO 3 ) 2 ·6H 2 O, 3.932gZr(NO 3 ) 4 ·3H 2 O and 1.386g Sc(NO 3 ) 3 (Purities are all AR grade) mixed together, add 90mL deionized water to prepare solution A; another 7.691g AR grade anhydrous K 2 CO 3 Dissolve in 95mL deionized water to prepare solution B; inject solution A and solution B into a reaction vessel at the same speed at 85°C (injection rate is 12mL / min), and keep stirring at 85°C Co-precipitation reaction, by regulating K 2 CO 3 Add the amount of aqueous solution to keep the pH value of the precipitation solution at 7.0 to 7.5. After the addition, continue to stir for 30 minutes and then stop stirring. Immediately filter the feed solution, and the obtained precipitate is washed with deionized water until it reaches K in the eluent. + The ion concentration is below 0.1ppm (detected by flame ion absorption method), and after filtration, the filter cake is dried at 115°C for 16 hours and roasted at 420°C for 3 hours to obtain the desired cat...
Embodiment 3
[0033] 6.979gNi(NO 3 ) 2 ·6H 2 O, 5.505gZr(NO 3 ) 4 ·3H 2 O and 0.462g Sc(NO 3 ) 3 (Purities are all AR grade) mixed together, add 90mL deionized water to prepare solution A; another 7.982g AR grade anhydrous K 2 CO 3 Dissolve in 95mL deionized water to prepare solution B; inject solution A and solution B into a reaction vessel at the same speed at 80°C (injection rate is 12mL / min), and keep stirring at 80°C Co-precipitation reaction, by regulating K 2 CO 3 Add the amount of aqueous solution to keep the pH value of the precipitation solution at 7.3 to 7.8. After the addition, continue to stir for 30 minutes and then stop stirring. Immediately filter the feed solution, and the obtained precipitate is washed with deionized water until it reaches K in the eluent. + The ion concentration is below 0.1ppm (detected by flame ion absorption method), and after filtration, the filter cake is dried at 105°C for 20 hours and roasted at 375°C for 5 hours to obtain the desired cat...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com