Copolymer, method of making the same, rubber composition and pneumatic tyre
A technology of polymers and copolymers, applied in the field of copolymers
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0118] In this example, the functionalization of styrene-butadiene rubber with 2-mercaptonaphthalene is illustrated to demonstrate the thiol-ene reaction.
[0119] To test the reactivity and reaction conditions of styrene-butadiene rubber in thiol-ene reactions, some reactions were performed with model thiols. The thiol of choice was 2-mercaptonaphthalene, available from Aldrich.
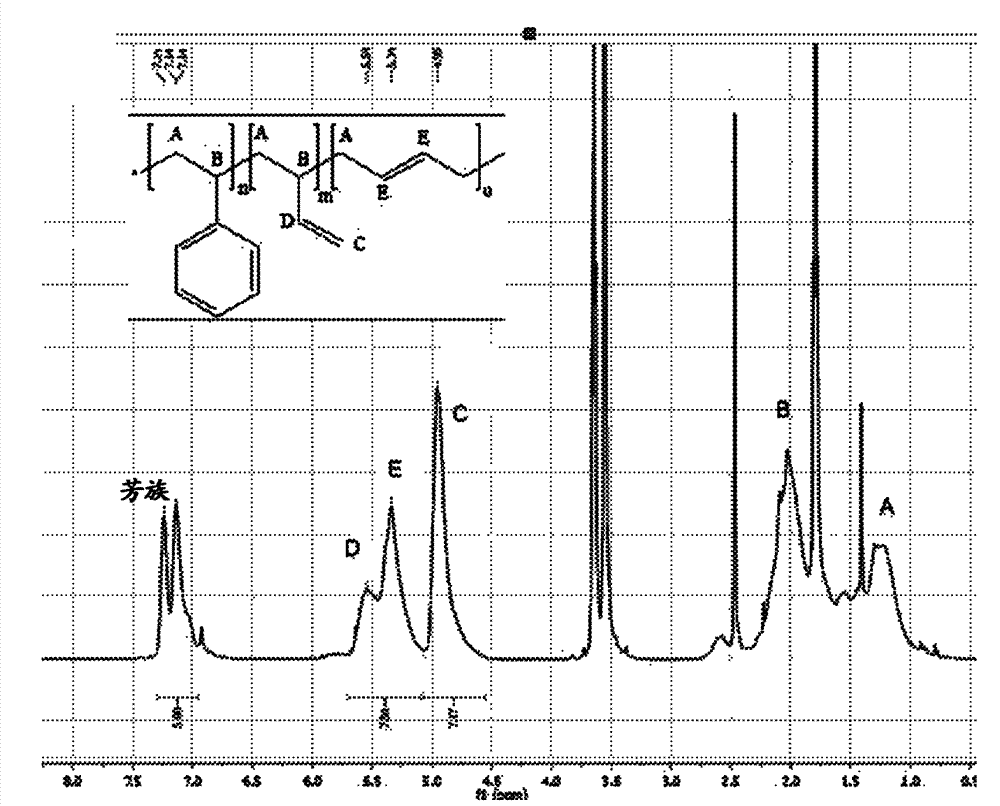
[0120] The properties of the styrene-butadiene rubber used are summarized in Table 1. Figure 1 shows the elastomeric 1 H-NMR spectrum.
[0121] Table 1
[0122] Mooney (average) 27.6 finish mooney 23.1 Tg(start) / ℃ -21.74 Tg(midpoint) / ℃ -17.52 FTIR styrene / % 25.392 FTIR Vinyl / % 47.506 FTIR cis / % 13.144 FTIR trans / % 13.058 Mn / g / mol 124122 Mw / g / mol 207982 Mz / g / mol 327454 PDI 1.67
[0123] Synthesis of 2-mercaptonaphthalene functionalized rubber
[0124] This compound was synthesized twice, once with AIBN as the init...
Embodiment 2
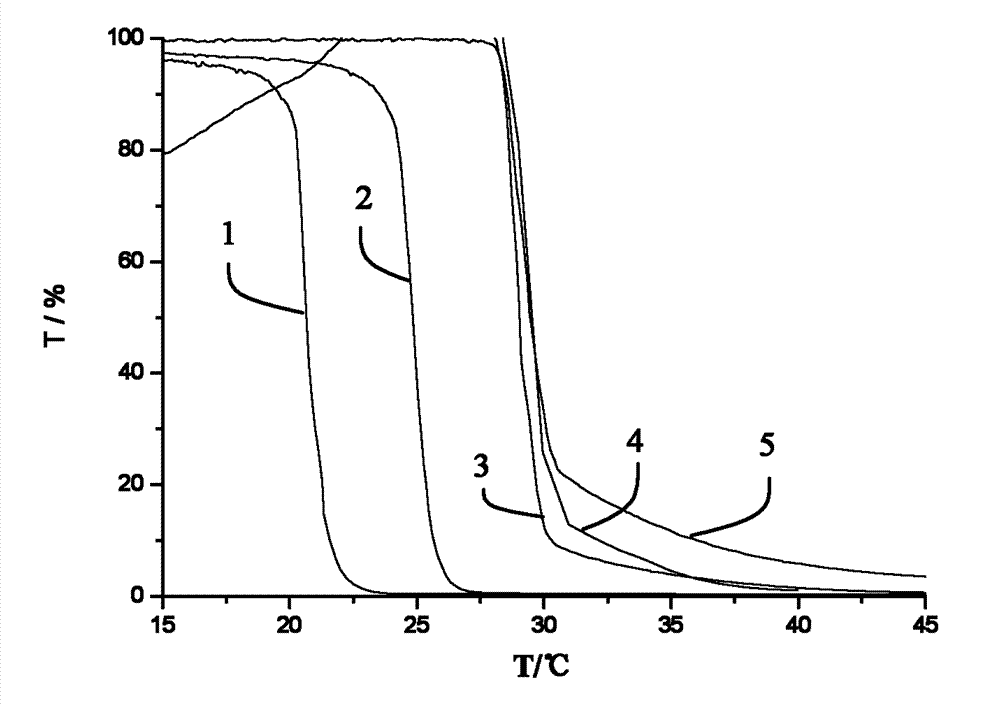
[0131] In this example the preparation of poly(N-isopropylacrylamide) or PNIPAM is illustrated.
[0132] Preparation of PNIPAM using RAFT polymerization. For this purpose, two alternative chain transfer agents (CTAs) were prepared: S-1-dodecyl-S-(α,α'-dimethyl-α"-acetic acid) trithiocarbonate (DMP ) and 4-cyano-4-dodecylthiocarbonylthio-4-methylbutanoic acid (CDSMB).
[0133]
[0134] The RAFT reaction scheme is as follows:
[0135]
[0136] Synthesis of Chain Transfer Agents
[0137] S-1-dodecyl-S-(α,α'-dimethyl-α"-acetic acid) trithiocarbonate (DMP)
[0138] S-1-dodecyl-S-(α,α'-dimethyl-α"-acetic acid) trithiocarbonate was synthesized using literature procedures [J. T. Lai, D. Filla, R. Shea, Macromolecules 2002, 35, 6754.]
[0139] Yield: 41%
[0140] 1 H-NMR (CDCl 3 / 300 MHz):δ[ppm]:0,85 (t, 3H), 1,16-1,47 (m, 20H), 1,71 (s, 6H), 3,26 (t, 2H), 13,05 (s, 1H)
[0141] 4-cyano-4-dodecylthiocarbonylthio-4-methylbutanoic acid (CDSMB)
[0142] 4-Cyano-4-...
Embodiment 3
[0162] In this example the functionalization of styrene-butadiene rubber with PNIPAM is illustrated.
[0163] Synthesis of Functionalized Rubber Elastomers
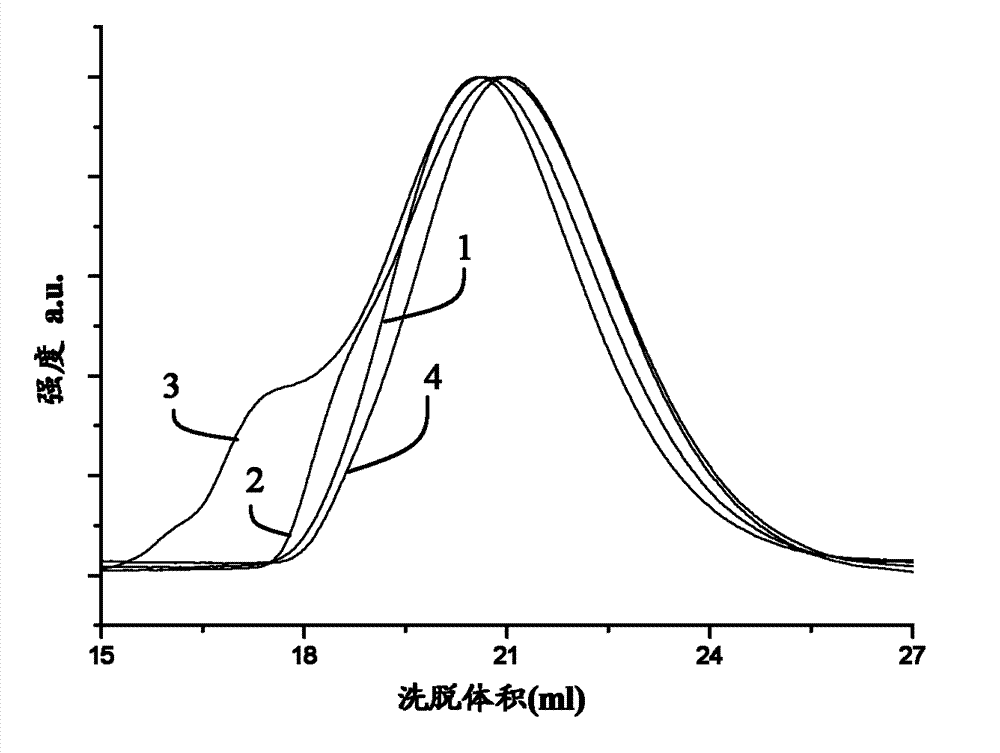
[0164] Functionalized elastomers were fabricated with the following general procedure: A solution of SBR, AIBN, and thiol in anhydrous THF was degassed under an argon atmosphere at room temperature for 2 hours. The exact amounts of educts for each reaction are shown in Table 4. The reaction mixture was then placed in a preheated oil bath at 70°C for at least 20 hours. To ensure that there were no free thiols in the reaction product, the product was dialyzed against THF for three days. After dialysis, the solvent was evaporated and the product was dried under vacuum. The results of the elemental analysis of the three functionalized elastomers are shown in Table 5 together with the calculated weight percent of PNIPAM in the resulting functionalized SBR.
[0165] SBR (1) and functionalized rubber (2) 1 The H-NMR spect...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com